Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma
- PMID: 32424805
- PMCID: PMC7467441
- DOI: 10.1007/s12325-020-01378-y
Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma
Abstract
Background: No trials have compared cabozantinib and regorafenib for the second-line treatment of advanced hepatocellular carcinoma (HCC).
Objectives: Conduct a matching-adjusted indirect comparison (MAIC) of the efficacy and safety of second-line cabozantinib and regorafenib in patients with advanced HCC and disease progression after prior sorafenib.
Methods: The CELESTIAL and RESORCE trials were used for indirect comparison of second-line cabozantinib and regorafenib in advanced HCC. Population-level data were available for RESORCE, individual patient data (IPD) for CELESTIAL. To align with RESORCE, the CELESTIAL population was limited to patients who received first-line sorafenib only. To minimize potential effect-modifying population differences, the CELESTIAL IPD were weighted to balance the distribution of clinically relevant baseline characteristics with those of RESORCE. Overall survival (OS) and progression-free survival (PFS) were evaluated for the matching-adjusted second-line CELESTIAL population and compared with those for RESORCE using weighted Kaplan-Meier curves and parametric modeling. Rates of grade 3/4 treatment-emergent adverse events (TEAEs) affecting > 5% of patients in any study arm were compared.
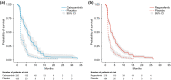
Results: In the matching-adjusted second-line populations (CELESTIAL, effective sample size = 266; RESORCE, n = 573), median (95% confidence interval) OS was similar for cabozantinib and regorafenib (11.4 [8.9-17.0] versus 10.6 [9.1-12.1] months; p = 0.3474, log-rank test). Median PFS was longer for cabozantinib than regorafenib (5.6 [4.9-7.3] versus 3.1 [2.8-4.2] months; p = 0.0005, log-rank test). There was a trend for lower rates of some grade 3/4 TEAEs with regorafenib than with cabozantinib, which may reflect the exclusion of sorafenib-intolerant patients from RESORCE but not from CELESTIAL, a difference that the MAIC methods could not remove. Only diarrhea rates were statistically significantly lower for regorafenib (p ≤ 0.001).
Conclusions: Cabozantinib may achieve similar OS and prolonged PFS compared with regorafenib in patients with progressive advanced HCC after prior sorafenib.
Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma. Video by Professor Katie Kelley.E. (MP4 420454 kb) Methods used in the matching-adjusted indirect comparison of cabozantinib versus regorafenib in patients with advanced hepatocellular carcinoma. A video. (MP4 160304 kb).
Keywords: CELESTIAL; Cabozantinib; Hepatocellular carcinoma (HCC); Indirect treatment comparison; Matching-adjusted indirect comparison (MAIC); RESORCE; Regorafenib; Second-line; Systemic therapy; Targeted therapy.
Plain language summary
Cabozantinib and regorafenib are treatments approved for some patients with advanced hepatocellular carcinoma (HCC), a type of liver cancer, after disease progression despite prior sorafenib treatment. Cabozantinib, regorafenib and sorafenib are tyrosine kinase inhibitors (TKIs), meaning that they slow cancer progression by targeting specific ways that tumors grow. Cabozantinib and regorafenib offer benefits to patients compared with placebo (i.e., no treatment) for those who have progressed despite sorafenib treatment. No clinical studies have compared cabozantinib and regorafenib directly. This study compared the efficacy and safety of cabozantinib and regorafenib using data from trials of each drug versus placebo: CELESTIAL for cabozantinib and RESORCE for regorafenib. These two trials were similar—both involved patients with progressive advanced HCC who had received previous cancer treatment. There were some important differences, but these were minimized using statistical methods (matching and adjustments/“weighting”) allowing outcomes to be meaningfully compared. One difference that could not be removed by the statistical methods was that patients who were intolerant to prior sorafenib were excluded from RESORCE but were eligible for the CELESTIAL trial. In the otherwise matched populations, treatment with cabozantinib was associated with similar overall survival and significantly longer progression-free survival than regorafenib. Rates of diarrhea were significantly lower for regorafenib than cabozantinib, suggesting that regorafenib may be better tolerated, but this may reflect the exclusion of sorafenib-intolerant patients from RESORCE. These findings cannot replace a head-to-head study, but may help in guiding decision-making between cabozantinib and regorafenib in patients with progressive advanced HCC after soraftenib treatment.
Figures


References
-
- Galle Peter R., Forner Alejandro, Llovet Josep M., Mazzaferro Vincenzo, Piscaglia Fabio, Raoul Jean-Luc, Schirmacher Peter, Vilgrain Valérie. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. Journal of Hepatology. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. - DOI - PubMed
-
- Bayer Schering Pharma. Nexavar® first FDA-approved drug therapy for liver cancer. 2007. https://www.investor.bayer.de/en/nc/news/archive/investor-news-2007/inve.... Accessed 17 April 2020.
-
- Kudo M. Targeted therapy for liver cancer: updated review in 2012. Curr Cancer Drug Targets. 2012;12(9):1062–1072. - PubMed

