p75NTR optimizes the osteogenic potential of human periodontal ligament stem cells by up-regulating α1 integrin expression
- PMID: 32424966
- PMCID: PMC7339167
- DOI: 10.1111/jcmm.15390
p75NTR optimizes the osteogenic potential of human periodontal ligament stem cells by up-regulating α1 integrin expression
Abstract
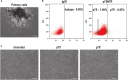
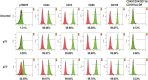
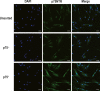
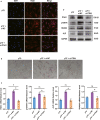
Human periodontal ligament stem cells (hPDLSCs) are a promising source in regenerative medicine. Due to the complexity and heterogeneity of hPDLSCs, it is critical to isolate homogeneous hPDLSCs with high regenerative potential. In this study, p75 neurotrophin receptor (p75NTR) was used to isolate p75NTR+ and p75NTR- hPDLSCs by fluorescence-activated cell sorting. Differences in osteogenic differentiation among p75NTR+ , p75NTR- and unsorted hPDLSCs were observed. Differential gene expression profiles between p75NTR+ and p75NTR- hPDLSCs were analysed by RNA sequencing. α1 Integrin (ITGA1) small interfering RNA and ITGA1-overexpressing adenovirus were used to transfect p75NTR+ and p75NTR- hPDLSCs. The results showed that p75NTR+ hPDLSCs demonstrated superior osteogenic capacity than p75NTR- and unsorted hPDLSCs. Differentially expressed genes between p75NTR+ and p75NTR- hPDLSCs were highly involved in the extracellular matrix-receptor interaction signalling pathway, and p75NTR+ hPDLSCs expressed higher ITGA1 levels than p75NTR- hPDLSCs. ITGA1 silencing inhibited the osteogenic differentiation of p75NTR+ hPDLSCs, while ITGA1 overexpression enhanced the osteogenic differentiation of p75NTR- hPDLSCs. These findings indicate that p75NTR optimizes the osteogenic potential of hPDLSCs by up-regulating ITGA1 expression, suggesting that p75NTR can be used as a novel cell surface marker to identify and purify hPDLSCs to promote their applications in regenerative medicine.
Keywords: cell surface marker; human periodontal ligament stem cells; osteogenic differentiation; regenerative medicine; signalling pathway.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures


References
-
- Trubiani O, Pizzicannella J, Caputi S, et al. Periodontal ligament stem cells: Current knowledge and future perspectives. Stem Cells Dev. 2019;28:995‐1003. - PubMed
-
- Seo B‐M, Miura M, Gronthos S, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149‐155. - PubMed
-
- Lei M, Li K, Li B, et al. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials. 2014;35:6332‐6343. - PubMed
-
- Liu J, Zhao Z, Ruan J, et al. Stem cells in the periodontal ligament differentiated into osteogenic, fibrogenic and cementogenic lineages for the regeneration of the periodontal complex. J Dent. 2020;92:103259‐. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

