A breeding strategy to identify modifiers of high genetic risk for methamphetamine intake
- PMID: 32424970
- PMCID: PMC7671946
- DOI: 10.1111/gbb.12667
A breeding strategy to identify modifiers of high genetic risk for methamphetamine intake
Abstract
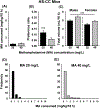
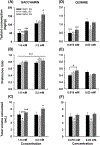
Trace amine-associated receptor 1 (Taar1) impacts methamphetamine (MA) intake. A mutant allele (Taar1m1J ) derived from the DBA/2J mouse strain codes for a non-functional receptor, and Taar1m1J/m1J mice consume more MA than mice possessing the reference Taar1+ allele. To study the impact of this mutation in a genetically diverse population, heterogeneous stock-collaborative cross (HS-CC) mice, the product of an eight-way cross of standard and wild-derived strains, were tested for MA intake. HS-CC had low MA intake, so an HS-CC by DBA/2J strain F2 intercross was created to transfer the mutant allele onto the diverse background, and used for selective breeding. To study residual variation in MA intake existing in Taar1m1J/m1J mice, selective breeding for higher (MAH) vs lower (MAL) MA intake was initiated from Taar1m1J/m1J F2 individuals; a control line of Taar1+/+ individuals (MAC) was retained. The lines were also examined for MA-induced locomotor and thermal responses, and fluid and tastant consumption. Taar1m1J/m1J F2 mice consumed significantly more MA than Taar1+/+ F2 mice. Response to selection was significant by generation 2 and there were corresponding differences in fluid consumed. Fluid consumption was not different in non-MA drinking studies. Taar1m1J/m1J genotype (MAL or MAH vs MAC mice) was associated with heighted MA locomotor and reduced hypothermic responses. MAL mice exhibited greater sensitization than MAH mice, but the selected lines did not consistently differ for thermal or tastant phenotypes. Residual variation among high-risk Taar1m1J/m1J mice appears to involve mechanisms associated with neuroadaptation to MA, but not sensitivity to hypothermic effects of MA.
Keywords: hypothermia; psychostimulant; quinine; saccharin; selective breeding; self-administration; sensitization; trace amine-associated receptor 1; two-bottle choice.
Published 2020. This article is a U.S. Government work and is in the public domain in the USA.
Figures







References
-
- NIDA. “Methamphetamine” National Institute on Drug Abuse, 16 Oct. 2019, https://www.drugabuse.gov/publications/research-reports/methamphetamine. Accessed 16 Dec. 2019NIDA.
-
- World Drug Report 2019. (United Nations publication, Sales No. E.19.XL.8)
-
- Bousman CA, Glatt SJ, Everall IP, Tsuang MT. Genetic association studies of methamphetamine use disorders: A systematic review and synthesis. Am J Med Genet. 2009;150B:1025–1049. - PubMed
-
- Iamjan SA, Thanoi S, Watiktinkorn P, Nudmamud-Thanoi S, Reynolds GP. BDNF (Val66Met) genetic polymorphism is associated with vulnerability for methamphetamine dependence. Pharmacogenomics. 2015;16:1541–1545. - PubMed
-
- Iamjan SA, Thanoi S, Watiktinkorn P, Reynolds GP, Nudmamud-Thanoi S. Genetic variation of GRIA3 gene is associated with vulnerability to methamphetamine dependence and its associated psychosis. J Psychopharmacol. 2018;32:309–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

