Mu opioid receptor knockout mouse: Phenotypes with implications on restless legs syndrome
- PMID: 32424971
- PMCID: PMC7430552
- DOI: 10.1002/jnr.24637
Mu opioid receptor knockout mouse: Phenotypes with implications on restless legs syndrome
Abstract
Restless legs syndrome (RLS) is characterized by an irresistible need to move the legs while sitting or lying at night with insomnia as a frequent consequence. Human RLS has been associated with abnormalities in the endogenous opioid system, the dopaminergic system, the iron regulatory system, anemia, and inflammatory and auto-immune disorders. Our previous work indicates that mice lacking all three subtypes of opioid receptors have a phenotype similar to that of human RLS. To study the roles of each opioid receptor subtype in RLS, we first used mu opioid receptor knockout (MOR KO) mice based on our earlier studies using postmortem brain and cell culture. The KO mice showed decreased hemoglobin, hematocrit, and red blood cells (RBCs), with an appearance of microcytic RBCs indicating anemia. Together with decreased serum iron and transferrin, but increased ferritin levels, the anemia is similar to that seen with chronic inflammation in humans. A decreased serum iron level was also observed in the wildtype mice treated with an MOR antagonist. Iron was increased in the liver and spleen of the KO mice. Normal circadian variations in the dopaminergic and serotoninergic systems were absent in the KO mice. The KO mice showed hyperactivity and increased thermal sensitivity in wakefulness primarily during what would normally be the sleep phase similar to that seen in human RLS. Deficits in endogenous opioid system transmission could predispose to anemia of inflammation and loss of circadian variations in dopaminergic or serotonergic systems, thereby contributing to an RLS-like phenotype.
Keywords: RRID:AB_10956736; RRID:AB_2261889; RRID:AB_621846; RRID:AB_621847; RRID:AB_631362; RRID:AB_641107; RRID:AB_668816; anemia of inflammation; circadian variations in monoamine systems; hyperactivity; iron deficiency; mu opioid receptor; thermosensory test.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
Figures


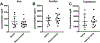


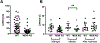
Similar articles
-
Review of the role of the endogenous opioid and melanocortin systems in the restless legs syndrome.Brain. 2024 Jan 4;147(1):26-38. doi: 10.1093/brain/awad283. Brain. 2024. PMID: 37633259 Free PMC article.
-
Hyperactivity, dopaminergic abnormalities, iron deficiency and anemia in an in vivo opioid receptors knockout mouse: Implications for the restless legs syndrome.Behav Brain Res. 2019 Nov 18;374:112123. doi: 10.1016/j.bbr.2019.112123. Epub 2019 Jul 31. Behav Brain Res. 2019. PMID: 31376441 Free PMC article.
-
Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in Btbd9 mutant mice.Hum Mol Genet. 2012 Sep 15;21(18):3984-92. doi: 10.1093/hmg/dds221. Epub 2012 Jun 7. Hum Mol Genet. 2012. PMID: 22678064 Free PMC article.
-
Iron, dopamine, genetics, and hormones in the pathophysiology of restless legs syndrome.J Neurol. 2017 Aug;264(8):1634-1641. doi: 10.1007/s00415-017-8431-1. Epub 2017 Feb 24. J Neurol. 2017. PMID: 28236139 Review.
-
[Pathophysiology of restless legs syndrome].Brain Nerve. 2009 May;61(5):523-32. Brain Nerve. 2009. PMID: 19514512 Review. Japanese.
Cited by
-
Putative Animal Models of Restless Legs Syndrome: A Systematic Review and Evaluation of Their Face and Construct Validity.Neurotherapeutics. 2023 Jan;20(1):154-178. doi: 10.1007/s13311-022-01334-4. Epub 2022 Dec 19. Neurotherapeutics. 2023. PMID: 36536233 Free PMC article.
-
Exploring the role of the endogenous opiate system in the pathogenesis of anemia in an opiate receptor knock-out model of Restless Legs Syndrome.Med Hypotheses. 2022 Oct;167:110941. doi: 10.1016/j.mehy.2022.110941. Epub 2022 Sep 5. Med Hypotheses. 2022. PMID: 36505961 Free PMC article.
-
Vitamin D and Restless Legs Syndrome: A Review of Current Literature.Tremor Other Hyperkinet Mov (N Y). 2023 Apr 6;13:12. doi: 10.5334/tohm.741. eCollection 2023. Tremor Other Hyperkinet Mov (N Y). 2023. PMID: 37034443 Free PMC article. Review.
-
Review of the role of the endogenous opioid and melanocortin systems in the restless legs syndrome.Brain. 2024 Jan 4;147(1):26-38. doi: 10.1093/brain/awad283. Brain. 2024. PMID: 37633259 Free PMC article.
-
Deficiency of Meis1, a transcriptional regulator, in mice and worms: Neurochemical and behavioral characterizations with implications in the restless legs syndrome.J Neurochem. 2020 Dec;155(5):522-537. doi: 10.1111/jnc.15177. Epub 2020 Sep 23. J Neurochem. 2020. PMID: 32910473 Free PMC article.
References
-
- Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, … Lee HB (2014). Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria--history, rationale, description, and significance. Sleep Med, 15(8), 860–873. doi:10.1016/j.sleep.2014.03.025 - DOI - PubMed
-
- Bachmann CG, Rolke R, Scheidt U, Stadelmann C, Sommer M, Pavlakovic G, … Paulus W (2010). Thermal hypoaesthesia differentiates secondary restless legs syndrome associated with small fibre neuropathy from primary restless legs syndrome. Brain, 133(Pt 3), 762–770. doi:10.1093/brain/awq026 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

