A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients
- PMID: 32425648
- PMCID: PMC7233230
- DOI: 10.1016/j.micinf.2020.05.008
A preliminary study on serological assay for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 238 admitted hospital patients
Abstract
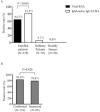
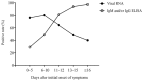
In this study, we aimed to evaluate the diagnostic value of serological assay for SARS-CoV-2. A newly-developed ELISA assay for IgM and IgG antibodies against N protein of SARS-CoV-2 was used to screen the serums of 238 admitted hospital patients between February 6 and February 14, 2020 with confirmed or suspected SARS-CoV-2. SARS-CoV-2 RNA was detected on pharyngeal swab specimens using real time RT-PCR. 194 (81.5%) of the serums were detected to be antibody (IgM and/or IgG) positive, significantly higher than the positive rate of viral RNA (64.3%). There was no difference in the positive rate of antibodies between the confirmed patients (83.0%, 127/153) and the suspected patients (78.8%, 67/85), whose nucleic acid tests were negative. The antibody positive rates were very low in the first five days after initial onset of symptoms, and then rapidly increased as the disease progressed. After 10 days, the antibody positive rates jumped from below 50% to over 80%. However, the positive rates of viral RNA maintained above 60% in the first 11 days after initial onset of symptoms, and then rapidly decreased. Overall, the suspected patients were most likely infected by SARS-CoV-2. Before the 11th day after initial onset of symptoms, nucleic acid test is key for confirmation of viral infection. The combination of serological assay can greatly improve the diagnostic efficacy. After the 11th day post-disease onset, the diagnosis for viral infection should be majorly dependent on serological assay.
Keywords: Diagnosis; Nucleic acid test; SARS-CoV-2; Serological assay.
Copyright © 2020 Institut Pasteur. Published by Elsevier Masson SAS. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that no conflict of interest exists.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

