Evaluation of the COVID19 ID NOW EUA assay
- PMID: 32425657
- PMCID: PMC7227587
- DOI: 10.1016/j.jcv.2020.104429
Evaluation of the COVID19 ID NOW EUA assay
Abstract
Background: The SARS-CoV-2 pandemic caused a major surge in needed diagnostic capacity. In response, many EUA assays have become available for clinical laboratories, and more recently, the point of care device, Abbott ID NOW.
Objectives: To determine the analytical performance of the ID NOW assay for detecting SARS-CoV-2.
Study design: Residual NP samples collected in viral transport media were tested by the ID NOW platform in two independent laboratories. Results were compared to either the CDC or New York EUA assays, which served as reference methods.
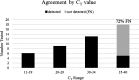
Results: Overall agreement of ID NOW was 78.7%. Sensitivity was 71.7% and specificity was 100%. Notably, all false-negative results correlated to those samples that were weakly positive.
Conclusions: ID NOW performs well for strong and moderately positive samples but has reduced sensitivity for weakly positive samples. This sensitivity, among other concerns, should be taken into consideration when using this test for patients with a low suspicion for COVID-19 disease.
Keywords: COVID-19; ID NOW; Point-of-Care; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
References
-
- ID NOW COVID-19 . Abbott Diagnostics Scarborough, Inc.; 2020. FDA Authorization Letter- March 27. https://www.fda.gov/medical-devices/emergency-situations-medical-devices... (Accessed 18 April 2020) Last updated: April 17, 2020.
-
- ID NOW COVID-19 . Abbott Diagnostics Scarborough, Inc.; 2020. Instructions for Use. EUA2000047. https://www.fda.gov/medical-devices/emergency-situations-medical-devices... (Accessed 18 April 18 2020) Last updated: April 17, 2020.
-
- Harrington A., Cox B., Snowdon J., Bakst J., Ley E., Grajales P., Maggiore J., Kahn S. Comparison of Abbott ID NOW and Abbott m2000 methods for the detection of SARS-Cov-2 from nasopharyngeal and nasal swabs from symptomatic patients. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00798-20. - DOI - PMC - PubMed
-
- Rhoads D.D., Cherian S.S., Roman K., Stempak L.M., Schmotzer C.L., Sadri N. Comparison of Abbott ID NOW, DiaSorin Simplexa and CDC FDA EUA methods for the detection of SARS-CoV-2 from nasopharyngeal and nasal swabs from individuals diagnosed with COVID-19. J. Clin. Microbiol. 2020 doi: 10.1128/JCM.00760-20. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous