A novel exopolysaccharide produced by Lactobacillus coryniformis NA-3 exhibits antioxidant and biofilm-inhibiting properties in vitro
- PMID: 32425737
- PMCID: PMC7217292
- DOI: 10.29219/fnr.v64.3744
A novel exopolysaccharide produced by Lactobacillus coryniformis NA-3 exhibits antioxidant and biofilm-inhibiting properties in vitro
Abstract
Background: Exopolysaccharides (EPSs) secreted from lactic acid bacteria are carbohydrate polymers with reported biological activities. In this study, we extracted and characterized the composition as well as antioxidant and biofilm-inhibitory properties of EPS from Lactobacillus coryniformis NA-3 isolated from northeast Chinese sauerkraut (Suan Cai).
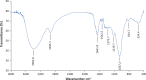
Methods: Lactobacillus coryniformis NA-3 was identified with 16S rDNA amplification and Neighbor Joining (NJ) phylogenetic analysis. EPS derived from Lactobacillus coryniformis NA-3 (EPS-NA3) was analyzed, including compositions by high-performance liquid chromatography (HPLC), functional groups by Fourier-transform infrared spectroscopy (FT-IR) and glycosidic bond configuration by Hydrogen-1 Nuclear Magnetic Resonance (1H NMR). Antioxidant activity of EPS was evaluated with hydroxyl and superoxide radical-scavenging. Anti-biofilm activities of EPS-NA3 were checked through inhibition and dispersion.
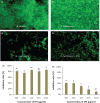
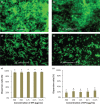
Results: The monosaccharide composition of EPS included α-rhamnose, α-mannose, α-galactose, and α-glucose in a ratio of 2.6:1.0:5.0:3.3. The free radical-scavenging abilities of EPS-NA3 were 37.77% ± 1.56% and 78.87% ± 3.07% on hydroxyl and superoxide reactive oxygen species respectively. Moreover, EPS-NA3 attenuated the formation of Bacillus cereus and Salmonella typhimurium biofilms by inhibition ratios of approximately 80% and 40% respectively. Additionally, treatment with EPS-NA3 dispersed established biofilms of B. cereus and S. typhimurium by approximately 90% and 20% respectively.
Conclusion: These results suggest that EPS-NA3 may be developed as antioxidant and anti-biofilm agents for industrial and clinical applications due to its capacity of scavenging free radicals, inhibition of bacterial biofilm formation, and dispersion of established biofilms.
Keywords: Lactobacillus coryniformis NA-3; anti-biofilm; antioxidant; dispersion; exopolysaccharide; inhibition.
© 2020 Xiaoqing Xu et al.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures



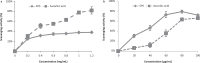
References
-
- Behare PV, Singh R, Kumar M, Prajapati JB, Singh RP. Exopolysaccharides of lactic acid bacteria: a review. J Food Sci Tech Mys 2009; 46(1): 1–11. doi: 10.1111/j.1750-3841.2008.01020.x - DOI
-
- Xu Y, Cui Y, Yue F, Liu L, Shan Y, Liu B, et al. . Exopolysaccharides produced by lactic acid bacteria and bifido bacteria: structures, physiochemical functions and applications in the food industry. Food Hydrocolloid 2019; 94: 475–499. doi: 10.1016/j.foodhyd.2019.03.032 - DOI
LinkOut - more resources
Full Text Sources

