Synaptic Plasticity on Motoneurons After Axotomy: A Necessary Change in Paradigm
- PMID: 32425754
- PMCID: PMC7203341
- DOI: 10.3389/fnmol.2020.00068
Synaptic Plasticity on Motoneurons After Axotomy: A Necessary Change in Paradigm
Abstract
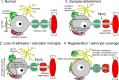
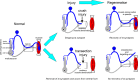
Motoneurons axotomized by peripheral nerve injuries experience profound changes in their synaptic inputs that are associated with a neuroinflammatory response that includes local microglia and astrocytes. This reaction is conserved across different types of motoneurons, injuries, and species, but also displays many unique features in each particular case. These reactions have been amply studied, but there is still a lack of knowledge on their functional significance and mechanisms. In this review article, we compiled data from many different fields to generate a comprehensive conceptual framework to best interpret past data and spawn new hypotheses and research. We propose that synaptic plasticity around axotomized motoneurons should be divided into two distinct processes. First, a rapid cell-autonomous, microglia-independent shedding of synapses from motoneuron cell bodies and proximal dendrites that is reversible after muscle reinnervation. Second, a slower mechanism that is microglia-dependent and permanently alters spinal cord circuitry by fully eliminating from the ventral horn the axon collaterals of peripherally injured and regenerating sensory Ia afferent proprioceptors. This removes this input from cell bodies and throughout the dendritic tree of axotomized motoneurons as well as from many other spinal neurons, thus reconfiguring ventral horn motor circuitries to function after regeneration without direct sensory feedback from muscle. This process is modulated by injury severity, suggesting a correlation with poor regeneration specificity due to sensory and motor axons targeting errors in the periphery that likely render Ia afferent connectivity in the ventral horn nonadaptive. In contrast, reversible synaptic changes on the cell bodies occur only while motoneurons are regenerating. This cell-autonomous process displays unique features according to motoneuron type and modulation by local microglia and astrocytes and generally results in a transient reduction of fast synaptic activity that is probably replaced by embryonic-like slow GABA depolarizations, proposed to relate to regenerative mechanisms.
Keywords: Ia afferent synapses; astrocytes; axotomy; microglia; motoneuron; regeneration; sensorimotor integration; synaptic plasticity.
Copyright © 2020 Alvarez, Rotterman, Akhter, Lane, English and Cope.
Figures

References
-
- Akhter E. T., Griffith R. W., English A. W., Alvarez F. J. (2019). Removal of the potassium chloride co-transporter from the somatodendritic membrane of axotomized motoneurons is independent of BDNF/TrkB signaling but is controlled by neuromuscular innervation. eNeuro 6:ENEURO.0172-19.2019. 10.1523/eneuro.0172-19.2019 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

