TSH Receptor Homodimerization in Regulation of cAMP Production in Human Thyrocytes in vitro
- PMID: 32425890
- PMCID: PMC7203478
- DOI: 10.3389/fendo.2020.00276
TSH Receptor Homodimerization in Regulation of cAMP Production in Human Thyrocytes in vitro
Abstract
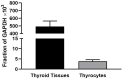
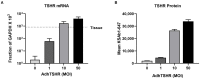
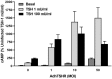
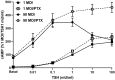
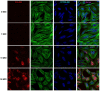
Thyrotropin hormone (TSH) was reported to exhibit biphasic regulation of cAMP production in human thyroid slices; specifically, upregulation at low TSH doses transitioning to inhibition at high doses. We observed this phenomenon in HEK293 cells overexpressing TSH receptors (TSHRs) but in only 25% of human thyrocytes (hThyros) in vitro. Because TSHR expression in hThyros in vitro was low, we tested the hypothesis that high, in situ levels of TSHRs were needed for biphasic cAMP regulation. We increased expression of TSHRs by infecting hThyros with adenoviruses expressing human TSHR (AdhTSHR), measured TSH-stimulated cAMP production and TSHR homodimerization. TSHR mRNA levels in hThyros in vitro were 100-fold lower than in human thyroid tissue. AdhTSHR infection increased TSHR mRNA expression to levels found in thyroid tissue and flow cytometry showed that cell-surface TSHRs increased more than 15-fold. Most uninfected hThyro preparations exhibited monotonic cAMP production. In contrast, most hThyro preparations infected with AdhTSHR expressing TSHR at in vivo levels exhibited biphasic TSH dose responses. Treatment of AdhTSHR-infected hThyros with pertussis toxin resulted in monotonic dose response curves demonstrating that lower levels of cAMP production at high TSH doses were mediated by Gi/Go proteins. Proximity ligation assays confirmed that AdhTSHR infection markedly increased the number of TSHR homodimers. We conclude that in situ levels of TSHRs as homodimers are needed for hThyros to exhibit biphasic TSH regulation of cAMP production.
Keywords: TSHR; cAMP production; inverted U-shaped dose response curve; phosphoinositide signaling; receptor homodimerization; thyrotropin receptor.
Copyright © 2020 Boutin, Krieger, Marcus-Samuels, Klubo-Gwiezdzinska, Neumann and Gershengorn.
Figures
References
-
- Corda D, Marcocci C, Kohn LD, Axelrod J, Luini A. Association of the changes in cytosolic Ca2+ and iodide efflux induced by thyrotropin and by the stimulation of alpha 1-adrenergic receptors in cultured rat thyroid cells. J Biol Chem. (1985) 260:9230–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

