Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years' follow-up of DEFINE, CONFIRM, and ENDORSE
- PMID: 32426039
- PMCID: PMC7222239
- DOI: 10.1177/1756286420915005
Safety and efficacy of delayed-release dimethyl fumarate in patients with relapsing-remitting multiple sclerosis: 9 years' follow-up of DEFINE, CONFIRM, and ENDORSE
Erratum in
-
Corrigendum to "Safety and Efficacy of Delayed-Release Dimethyl Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis: 9 Years' Follow-up of DEFINE, CONFIRM, and ENDORSE".Ther Adv Neurol Disord. 2020 Oct 21;13:1756286420968357. doi: 10.1177/1756286420968357. eCollection 2020. Ther Adv Neurol Disord. 2020. PMID: 35173806 Free PMC article.
Abstract
Introduction: We report safety and efficacy in patients treated with dimethyl fumarate (DMF) for ~9 years in ENDORSE. Lymphocyte analysis data are also reported.
Methods: Incidence of serious adverse events (SAEs), discontinuations due to adverse events (AEs), annualized relapse rate (ARR) and Expanded Disability Status Scale (EDSS) score were assessed. Patients were treated with DMF 240 mg twice daily (BID): placebo (PBO)/DMF (PBO for years 0-2 /DMF for years 3-9) or continuous (DMF/DMF) treatment; newly diagnosed patients were included. Annual magnetic resonance imaging (MRI) was evaluated in patients from the MRI cohort of DEFINE/CONFIRM. For the lymphocyte analysis, data from first DMF exposure were analyzed.
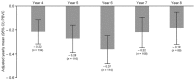
Results: Of 2079 DEFINE/CONFIRM completers, 1736 enrolled and received ⩾1 dose of DMF. The MRI cohort included 530 patients. In the overall population, 527 (30%) patients experienced SAEs; most were fall and urinary tract infection. Over 9 years on DMF treatment, adjusted ARR remained low (⩽0.20). In patients treated with PBO in years 0-2, decreased ARR was apparent as early as year 3. Of DMF/DMF and PBO/DMF patients, 73% and 74%, respectively, had no 24-week confirmed disability progression. Most patients (~70%) had no new T1 or new/newly enlarging T2 lesions compared with previous MRI scans after 7 years treatment with DMF; the annual number of new T1 hypointense lesions and new/newly enlarging T2 hyperintense lesions were 0.6-0.8 and 0.9-2.0, respectively. Mean percentage brain volume change from ENDORSE baseline (6 years treatment in ENDORSE) was -1.32% (range -1.60% to -1.05%). Of the 2513 patients with lymphocyte assessments, 2470 had ⩾1 post-baseline measurement, 53 developed severe prolonged lymphopenia and were followed for up to 11 years; incidence of serious infection was not higher than in patients with absolute lymphocyte count (ALC) always ⩾ lower limit of normal (LLN). In patients with lymphopenia while on DMF and ALC < 0.91 × 109/L at discontinuation (n = 138), median time to ALC ⩾ LLN was 7 weeks post-discontinuation.
Conclusions: Sustained safety and efficacy of DMF was observed in patients continuing on treatment for up to 11 years, supporting DMF as a long-term treatment option for patients with RRMS.
Trial registration: ClinicalTrials.gov identifiers, NCT00835770 (ENDORSE); NCT00420212 (DEFINE); NCT00451451 (CONFIRM).
Keywords: delayed-release dimethyl fumarate; efficacy; multiple sclerosis; newly diagnosed; safety.
© The Author(s), 2020.
Conflict of interest statement
Conflict of interest statement: RG reports honoraria/research support from Bayer, Biogen, Merck Serono, Novartis, and Teva Neuroscience; and compensation from Sage for serving as editor of Therapeutic Advances in Neurological Disorders. DLA reports consultant fees and/or grants from Acorda, Adelphi, Alkermes, Biogen, Celgene, Genentech, Genzyme, Hoffman-La Roche, Immune Tolerance Network, Immunotec, MedDay, Novartis, Pfizer, Receptos, Roche, Sanofi-Aventis, Canadian Institutes of Health Research, MS Society of Canada, and International Progressive MS Alliance; and equity interest in NeuroRx Research. AB-O reports speaker/consulting fees/grant support from Atara Biotherapeutics, Biogen, Celgene/Receptos, Genentech/Roche, GlaxoSmithKline, MedImmune, Merck/EMD Serono, Novartis, and Sanofi-Genzyme. RJF reports consulting fees from Biogen, MedDay, Novartis, Questcor, Teva, and XenoPort; advisory committees for Biogen and Novartis; and research grant funding from Novartis. LK reports the following, which were payed to his institution in the past 3 years and used exclusively for research support: steering committee, advisory board and consultancy fees from Actelion, Alkermes, Almirall, Bayer, Biogen, Celgene/Receptos, df-mp, EXCEMED, GeNeuro SA, Genzyme, Japan Tobacco, Merck, Minoryx, Mitsubishi Pharma, Novartis, Roche, Sanofi-Aventis, Santhera, Teva and Vianex; and license fees for Neurostatus-UHB products; research performed at his institution has been supported by grants from Bayer, Biogen, Novartis, the European Union, Innoswiss, Roche Research Foundations, the Swiss MS Society and the Swiss National Research Foundation. CC, BP, and CM are employees of, and hold stock/stock options in, Biogen.
Figures






References
-
- Biogen Inc. Tecfidera® (dimethyl fumarate) delayed-release capsules, for oral use, https://www.tecfidera.com/content/dam/commercial/multiple-sclerosis/tecf... (2019).
-
- European Medicines Agency. Tecfidera 120 mg gastro-resistant hard capsules, https://www.ema.europa.eu/documents/product-information/tecfidera-epar-p....
-
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012; 367: 1098–1107. - PubMed
-
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012; 367: 1087–1097. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

