Machine Learning Classification of Articular Cartilage Integrity Using Near Infrared Spectroscopy
- PMID: 32426059
- PMCID: PMC7225230
- DOI: 10.1007/s12195-020-00612-5
Machine Learning Classification of Articular Cartilage Integrity Using Near Infrared Spectroscopy
Abstract
Introduction: Assessment of cartilage integrity during arthroscopy is limited by the subjective visual nature of the technique. To address this shortcoming in diagnostic evaluation of articular cartilage, near infrared spectroscopy (NIRS) has been proposed. In this study, we evaluated the capacity of NIRS, combined with machine learning techniques, to classify cartilage integrity.
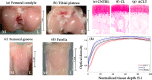
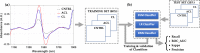
Methods: Rabbit (n = 14) knee joints with artificial injury, induced via unilateral anterior cruciate ligament transection (ACLT), and the corresponding contra-lateral (CL) joints, including joints from separate non-operated control (CNTRL) animals (n = 8), were used. After sacrifice, NIR spectra (1000-2500 nm) were acquired from different anatomical locations of the joints (n TOTAL = 313: n CNTRL = 111, n CL = 97, n ACLT = 105). Machine and deep learning methods (support vector machines-SVM, logistic regression-LR, and deep neural networks-DNN) were then used to develop models for classifying the samples based solely on their NIR spectra.
Results: The results show that the model based on SVM is optimal of distinguishing between ACLT and CNTRL samples (ROC_AUC = 0.93, kappa = 0.86), LR is capable of distinguishing between CL and CNTRL samples (ROC_AUC = 0.91, kappa = 0.81), while DNN is optimal for discriminating between the different classes (multi-class classification, kappa = 0.48).
Conclusion: We show that NIR spectroscopy, when combined with machine learning techniques, is capable of holistic assessment of cartilage integrity, with potential for accurately distinguishing between healthy and diseased cartilage.
Keywords: Cartilage; Classification; Deep learning; Machine learning; Near infrared spectroscopy; Osteoarthritis.
© The Author(s) 2020.
Figures



References
-
- Afara I, Prasadam I, Crawford R, Xiao Y, Oloyede A. Non-destructive evaluation of articular cartilage defects using near-infrared (NIR) spectroscopy in osteoarthritic rat models and its direct relation to Mankin score. Osteoarthr. Cartil. 2012;20:1367–1373. doi: 10.1016/j.joca.2012.07.007. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

