Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma
- PMID: 32426105
- PMCID: PMC7216502
- DOI: 10.1186/s13578-020-00425-z
Ghrelin, a novel therapy, corrects cytokine and NF-κB-AKT-MAPK network and mitigates intestinal injury induced by combined radiation and skin-wound trauma
Abstract
Background: Compared to radiation injury alone (RI), radiation injury combined wound (CI) further enhances acute radiation syndrome and subsequently mortality. We previously reported that therapy with Ghrelin, the 28-amino-acid-peptide secreted from the stomach, significantly increased 30-day survival and mitigated hematopoietic death by enhancing and sustaining granulocyte-colony stimulating factor (G-CSF) and keratinocyte chemoattractant (KC) in the blood and bone marrow; increasing circulating white blood cell depletion; inhibiting splenocytopenia; and accelerating skin-wound healing on day 30 after CI. Herein, we aimed to study the efficacy of Ghrelin on intestinal injury at early time points after CI.
Methods: B6D2F1/J female mice were exposed to 60Co-γ-photon radiation (9.5 Gy, 0.4 Gy/min, bilateral), followed by 15% total-body-surface-area skin wounds. Several endpoints were measured: at 4-5 h and on days 1, 3, 7, and 15.
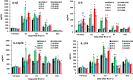
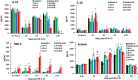
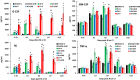
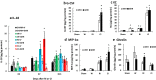
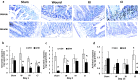
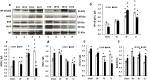
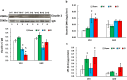
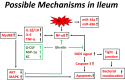
Results: Ghrelin therapy mitigated CI-induced increases in IL-1β, IL-6, IL-17A, IL-18, KC, and TNF-α in serum but sustained G-CSF, KC and MIP-1α increases in ileum. Histological analysis of ileum on day 15 showed that Ghrelin treatment mitigated ileum injury by increasing villus height, crypt depth and counts, as well as decreasing villus width and mucosal injury score. Ghrelin therapy increased AKT activation and ERK activation; suppressed JNK activation and caspase-3 activation in ileum; and reduced NF-κB, iNOS, BAX and Bcl-2 in ileum. This therapy recovered the tight junction protein and mitigated bacterial translocation and lipopolysaccharides levels. The results suggest that the capacity of Ghrelin therapy to reduce CI-induced ileum injury is mediated by a balanced NF-κB-AKT-MAPK network that leads to homeostasis of pro-inflammatory and anti-inflammatory cytokines.
Conclusions: Our novel results are the first to suggest that Ghrelin therapy effectively decreases intestinal injury after CI.
Keywords: AKT; Apoptosis; BAX; Bacteria; Bcl-2; Caspase; ERK; GI; Ghrelin; Ionizing radiation; JNK; MAPK; NF-κB; Skin wound; Tight junction; iNOS.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThe authors declare that they have no conflict of interests.
Figures



Similar articles
-
Ghrelin therapy mitigates bone marrow injury and splenocytopenia by sustaining circulating G-CSF and KC increases after irradiation combined with wound.Cell Biosci. 2018 Apr 5;8:27. doi: 10.1186/s13578-018-0225-3. eCollection 2018. Cell Biosci. 2018. PMID: 29632660 Free PMC article.
-
A Combined Therapy of Pegylated G-CSF with Ciprofloxacin Mitigates Damage Induced by Lethal Ionizing Radiation to the Bone Marrow, Spleen, and Ileum by Increasing AKT Activation but Decreasing IL-18, C3, and miR-34a.Radiat Res. 2025 May 1;203(5):341-356. doi: 10.1667/RADE-24-00266.1. Radiat Res. 2025. PMID: 40181563
-
A novel therapy, using Ghrelin with pegylated G-CSF, inhibits brain hemorrhage from ionizing radiation or combined radiation injury.Pharm Pharmacol Int J. 2019;7(3):133-145. doi: 10.15406/ppij.2019.07.00243. Epub 2019 Jun 26. Pharm Pharmacol Int J. 2019. PMID: 34368440 Free PMC article.
-
Female Mice are More Resistant to the Mixed-Field (67% Neutron + 33% Gamma) Radiation-Induced Injury in Bone Marrow and Small Intestine than Male Mice due to Sustained Increases in G-CSF and the Bcl-2/Bax Ratio and Lower miR-34a and MAPK Activation.Radiat Res. 2022 Aug 1;198(2):120-133. doi: 10.1667/RADE-21-00201.1. Radiat Res. 2022. PMID: 35452510 Free PMC article.
-
Hemorrhage enhances cytokine, complement component 3, and caspase-3, and regulates microRNAs associated with intestinal damage after whole-body gamma-irradiation in combined injury.PLoS One. 2017 Sep 21;12(9):e0184393. doi: 10.1371/journal.pone.0184393. eCollection 2017. PLoS One. 2017. PMID: 28934227 Free PMC article.
Cited by
-
Transcriptomics of Wet Skin Biopsies Predict Early Radiation-Induced Hematological Damage in a Mouse Model.Genes (Basel). 2022 Mar 18;13(3):538. doi: 10.3390/genes13030538. Genes (Basel). 2022. PMID: 35328091 Free PMC article.
-
Fetal Muse-based therapy prevents lethal radio-induced gastrointestinal syndrome by intestinal regeneration.Stem Cell Res Ther. 2023 Aug 11;14(1):201. doi: 10.1186/s13287-023-03425-1. Stem Cell Res Ther. 2023. PMID: 37568164 Free PMC article.
-
Mesenchymal Stem Cells for Mitigating Radiotherapy Side Effects.Cells. 2021 Feb 1;10(2):294. doi: 10.3390/cells10020294. Cells. 2021. PMID: 33535574 Free PMC article. Review.
-
Radiation-Induced Intestinal Injury: Injury Mechanism and Potential Treatment Strategies.Toxics. 2023 Dec 10;11(12):1011. doi: 10.3390/toxics11121011. Toxics. 2023. PMID: 38133412 Free PMC article. Review.
-
Effects of combined ciprofloxacin and Neulasta therapy on intestinal pathology and gut microbiota after high-dose irradiation in mice.Front Public Health. 2024 May 14;12:1365161. doi: 10.3389/fpubh.2024.1365161. eCollection 2024. Front Public Health. 2024. PMID: 38807988 Free PMC article.
References
-
- DiCarlo AL, Hatchett RJ, Kaminski JM, Ledney GD, Pellmar TC, Okunieff P, Ramakrishnan N. Medical countermeasures for radiation combined injury: radiation with burn, blast, trauma and/or sepsis. Report of an NIAID Workshop, March 26–27. Radiat Res. 2008;169:712–721. doi: 10.1667/RR1295.1. - DOI - PMC - PubMed
-
- Iijima S. Pathology of atomic bomb casualties. Acta Pathol Jpn. 1982;32(Suppl. 2):237–270. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

