Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells
- PMID: 32426106
- PMCID: PMC7218515
- DOI: 10.1186/s13578-020-00426-y
Synergies in exosomes and autophagy pathways for cellular homeostasis and metastasis of tumor cells
Abstract
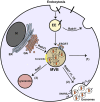
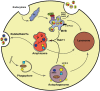
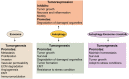
Background: Eukaryotic cells demonstrate two tightly linked vesicular transport systems, comprising intracellular vesicle transport and extracellular vesicle transport system. Intracellular transport vesicles can translocate biomolecules between compartments inside the cell, for example, proteins from the rough endoplasmic reticulum to the Golgi apparatus. Whereas, the secreted vesicles so-called extracellular vesicles facilitate the transport of biomolecules, for example, nucleic acids, proteins and lipids between cells. Vesicles can be formed during the process of endocytosis or/and autophagy and not only act as mediators of intra- and inter-cellular communication but also represent pathological conditions of cells or tissues.
Methods: In this review, we searched articles in PubMed, published between 2000 and 2020, with following terms: autophagy, autophagocytosis, transport vesicles, lysosomes, endosomes, exocytosis, exosomes, alone or in different combinations. The biological functions that were selected based on relevancy to our topic include cellular homeostasis and tumorigenesis.
Results: The searched literature shows that there is a high degree of synergies between exosome biogenesis and autophagy, which encompass endocytosis and endosomes, lysosomes, exocytosis and exosomes, autophagocytosis, autophagosomes and amphisomes. These transport systems not only maintain cellular homeostasis but also operate synergically against fluctuations in the external and internal environment such as during tumorigenesis and metastasis. Additionally, exosomal and autophagic proteins may serve as cancer diagnosis approaches.
Conclusion: Exosomal and autophagy pathways play pivotal roles in homeostasis and metastasis of tumor cells. Understanding the crosstalk between endomembrane organelles and vesicular trafficking may expand our insight into cooperative functions of exosomal and autophagy pathways during disease progression and may help to develop effective therapies against lysosomal diseases including cancers and beyond.
Keywords: Autophagosomes; Autophagy; Autophagy associated tumorigenesis; Autophagy-mediated exosomes; Cancer cell metastasis; Endosomes; Extracellular vesicles.
© The Author(s) 2020.
Conflict of interest statement
Competing interestsThere is no any conflict of interest by the authors competing interests.
Figures

References
-
- Tokarev AA, Alfonso A, Segev N. Overview of intracellular compartments and trafficking pathways. Trafficking Inside Cells: Springer; 2009. pp. 3–14.
Publication types
LinkOut - more resources
Full Text Sources

