Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation
- PMID: 32426347
- PMCID: PMC7204401
- DOI: 10.3389/fbioe.2020.00373
Stiffness Matters: Fine-Tuned Hydrogel Elasticity Alters Chondrogenic Redifferentiation
Abstract
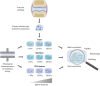
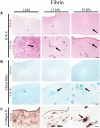
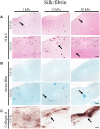
Biomechanical cues such as shear stress, stretching, compression, and matrix elasticity are vital in the establishment of next generation physiological in vitro tissue models. Matrix elasticity, for instance, is known to guide stem cell differentiation, influence healing processes and modulate extracellular matrix (ECM) deposition needed for tissue development and maintenance. To better understand the biomechanical effect of matrix elasticity on the formation of articular cartilage analogs in vitro, this study aims at assessing the redifferentiation capacity of primary human chondrocytes in three different hydrogel matrices of predefined matrix elasticities. The hydrogel elasticities were chosen to represent a broad spectrum of tissue stiffness ranging from very soft tissues with a Young's modulus of 1 kPa up to elasticities of 30 kPa, representative of the perichondral-space. In addition, the interplay of matrix elasticity and transforming growth factor beta-3 (TGF-β3) on the redifferentiation of primary human articular chondrocytes was studied by analyzing both qualitative (viability, morphology, histology) and quantitative (RT-qPCR, sGAG, DNA) parameters, crucial to the chondrotypic phenotype. Results show that fibrin hydrogels of 30 kPa Young's modulus best guide chondrocyte redifferentiation resulting in a native-like morphology as well as induces the synthesis of physiologic ECM constituents such as glycosaminoglycans (sGAG) and collagen type II. This comprehensive study sheds light onto the mechanobiological impact of matrix elasticity on formation and maintenance of articular cartilage and thus represents a major step toward meeting the need for advanced in vitro tissue models to study both re- and degeneration of articular cartilage.
Keywords: 3D cell culture; Young’s modulus; cartilage; chondrocytes; extracellular matrix; hydrogel.
Copyright © 2020 Bachmann, Spitz, Schädl, Teuschl, Redl, Nürnberger and Ertl.
Figures


References
-
- Carter D. R., Beaupre G. S., Wong M., Smith R. L., Andriacchi T. P., Schurman D. J. (2004). The mechanobiology of articular cartilage development and degeneration. Clin. Orthop. Relat. Res. 427 S69–S77. - PubMed
LinkOut - more resources
Full Text Sources

