Cancer Stem Cell Plasticity - A Deadly Deal
- PMID: 32426371
- PMCID: PMC7203492
- DOI: 10.3389/fmolb.2020.00079
Cancer Stem Cell Plasticity - A Deadly Deal
Abstract
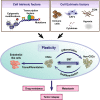
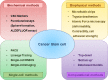
Intratumoral heterogeneity is a major ongoing challenge in the effective therapeutic targeting of cancer. Accumulating evidence suggests that a fraction of cells within a tumor termed Cancer Stem Cells (CSCs) are primarily responsible for this diversity resulting in therapeutic resistance and metastasis. Adding to this complexity, recent studies have shown that there can be different subpopulations of CSCs with varying biochemical and biophysical traits resulting in varied dissemination and drug-resistance potential. Moreover, cancer cells can exhibit a high level of plasticity or the ability to dynamically switch between CSC and non-CSC states or among different subsets of CSCs. In addition, CSCs also display extensive metabolic plasticity. The molecular mechanisms underlying these different interconnected axes of plasticity has been under extensive investigation and the trans-differentiation process of Epithelial to Mesenchymal transition (EMT) has been identified as a major contributing factor. Besides genetic and epigenetic factors, CSC plasticity is also shaped by non-cell-autonomous effects such as the tumor microenvironment (TME). In this review, we discuss the latest developments in decoding mechanisms and implications of CSC plasticity in tumor progression at biochemical and biophysical levels, and the latest in silico approaches being taken for characterizing cancer cell plasticity. These efforts can help improve existing therapeutic approaches by taking into consideration the contribution of cellular plasticity/heterogeneity in enabling drug resistance.
Keywords: cancer stem cells; epithelial-mesenchymal transition; metabolic plasticity; metastasis; microenvironment; plasticity.
Copyright © 2020 Thankamony, Saxena, Murali, Jolly and Nair.
Figures

References
-
- Akrap N., Andersson D., Bom E., Gregersson P., Stahlberg A., Landberg G. (2016). Identification of distinct breast cancer stem cell populations based on single-cell analyses of functionally enriched stem and progenitor pools. Stem Cell Rep. 6 121–136. 10.1016/j.stemcr.2015.12.006 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

