Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer's disease
- PMID: 32426454
- PMCID: PMC7159908
- DOI: 10.1126/sciadv.aaz2387
Aβ deposition is associated with increases in soluble and phosphorylated tau that precede a positive Tau PET in Alzheimer's disease
Abstract
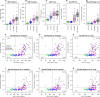
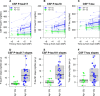
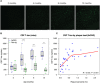
The links between β-amyloid (Aβ) and tau in Alzheimer's disease are unclear. Cognitively unimpaired persons with signs of Aβ pathology had increased cerebrospinal fluid (CSF) phosphorylated tau (P-tau181 and P-tau217) and total-tau (T-tau), which increased over time, despite no detection of insoluble tau aggregates [normal Tau positron emission tomography (PET)]. CSF P-tau and T-tau started to increase before the threshold for Amyloid PET positivity, while Tau PET started to increase after Amyloid PET positivity. Effects of Amyloid PET on Tau PET were mediated by CSF P-tau, and high CSF P-tau predicted increased Tau PET rates. Individuals with MAPT mutations and signs of tau deposition (but without Aβ pathology) had normal CSF P-tau levels. In 5xFAD mice, CSF tau increased when Aβ aggregation started. These results show that Aβ pathology may induce changes in soluble tau release and phosphorylation, which is followed by tau aggregation several years later in humans.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Goedert M., Tau filaments in neurodegenerative diseases. FEBS Lett. 592, 2383–2391 (2018). - PubMed
-
- Cicognola C., Brinkmalm G., Wahlgren J., Portelius E., Gobom J., Cullen N. C., Hansson O., Parnetti L., Constantinescu R., Wildsmith K., Chen H.-H., Beach T. G., Lashley T., Zetterberg H., Blennow K., Höglund K., Novel tau fragments in cerebrospinal fluid: Relation to tangle pathology and cognitive decline in Alzheimer’s disease. Acta Neuropathol. 137, 279–296 (2019). - PMC - PubMed
-
- Maia L. F., Kaeser S. A., Reichwald J., Hruscha M., Martus P., Staufenbiel M., Jucker M., Changes in amyloid-β and Tau in the cerebrospinal fluid of transgenic mice overexpressing amyloid precursor protein. Sci. Transl. Med. 5, 194re2 (2013). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

