Nanoparticle elasticity regulates phagocytosis and cancer cell uptake
- PMID: 32426455
- PMCID: PMC7164958
- DOI: 10.1126/sciadv.aaz4316
Nanoparticle elasticity regulates phagocytosis and cancer cell uptake
Abstract
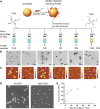
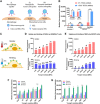
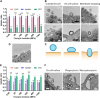
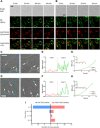
The ability of cells to sense external mechanical cues is essential for their adaptation to the surrounding microenvironment. However, how nanoparticle mechanical properties affect cell-nanoparticle interactions remains largely unknown. Here, we synthesized a library of silica nanocapsules (SNCs) with a wide range of elasticity (Young's modulus ranging from 560 kPa to 1.18 GPa), demonstrating the impact of SNC elasticity on SNC interactions with cells. Transmission electron microscopy revealed that the stiff SNCs remained spherical during cellular uptake. The soft SNCs, however, were deformed by forces originating from the specific ligand-receptor interaction and membrane wrapping, which reduced their cellular binding and endocytosis rate. This work demonstrates the crucial role of the elasticity of nanoparticles in modulating their macrophage uptake and receptor-mediated cancer cell uptake, which may shed light on the design of drug delivery vectors with higher efficiency.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- Gillespie P. G., Walker R. G., Molecular basis of mechanosensory transduction. Nature 413, 194–202 (2001). - PubMed
-
- Qiu Y., Brown A. C., Myers D. R., Sakurai Y., Mannino R. G., Tran R., Ahn B., Hardy E. T., Kee M. F., Kumar S., Bao G., Barker T. H., Lam W. A., Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. U.S.A. 111, 14430–14435 (2014). - PMC - PubMed
-
- Vogel V., Sheetz M., Local force and geometry sensing regulate cell functions. Nat. Rev. Mol. Cell Biol. 7, 265–275 (2006). - PubMed
-
- Discher D. E., Janmey P., Wang Y.-L., Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

