Wireless smart contact lens for diabetic diagnosis and therapy
- PMID: 32426469
- PMCID: PMC7182412
- DOI: 10.1126/sciadv.aba3252
Wireless smart contact lens for diabetic diagnosis and therapy
Abstract
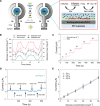
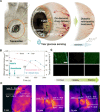
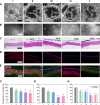
A smart contact lens can be used as an excellent interface between the human body and an electronic device for wearable healthcare applications. Despite wide investigations of smart contact lenses for diagnostic applications, there has been no report on electrically controlled drug delivery in combination with real-time biometric analysis. Here, we developed smart contact lenses for both continuous glucose monitoring and treatment of diabetic retinopathy. The smart contact lens device, built on a biocompatible polymer, contains ultrathin, flexible electrical circuits and a microcontroller chip for real-time electrochemical biosensing, on-demand controlled drug delivery, wireless power management, and data communication. In diabetic rabbit models, we could measure tear glucose levels to be validated by the conventional invasive blood glucose tests and trigger drugs to be released from reservoirs for treating diabetic retinopathy. Together, we successfully demonstrated the feasibility of smart contact lenses for noninvasive and continuous diabetic diagnosis and diabetic retinopathy therapy.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Chu B., Burnett W., Chung J. W., Bao Z., Bring on the bodyNET. Nature 549, 328–330 (2017). - PubMed
-
- Someya T., Bao Z., Malliaras G. G., The rise of plastic bioelectronics. Nature 540, 379–385 (2016). - PubMed
-
- Yamagishi K., Kirino I., Takahashi I., Amano H., Takeoka S., Morimoto Y., Fujie T., Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy. Nat. Biomed. Eng. 3, 27–36 (2019). - PubMed
-
- Lee H., Choi T. K., Lee Y. B., Cho H. R., Ghaffari R., Wang L., Choi H. J., Chung T. D., Lu N., Hyeon T., Choi S. H., Kim D.-H., A graphene-based electrochemical device with thermoresponsive microneedles for diabetes monitoring and therapy. Nat. Nanotechnol. 11, 566–572 (2016). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

