A novel proangiogenic B cell subset is increased in cancer and chronic inflammation
- PMID: 32426497
- PMCID: PMC7220305
- DOI: 10.1126/sciadv.aaz3559
A novel proangiogenic B cell subset is increased in cancer and chronic inflammation
Abstract
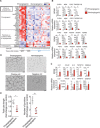
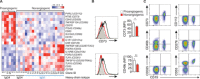
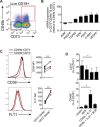
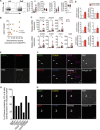
B cells contribute to immune responses through the production of immunoglobulins, antigen presentation, and cytokine production. Several B cell subsets with distinct functions and polarized cytokine profiles have been reported. In this study, we used transcriptomics analysis of immortalized B cell clones to identify an IgG4+ B cell subset with a unique function. These B cells are characterized by simultaneous expression of proangiogenic cytokines including VEGF, CYR61, ADM, FGF2, PDGFA, and MDK. Consequently, supernatants from these clones efficiently promote endothelial cell tube formation. We identified CD49b and CD73 as surface markers identifying proangiogenic B cells. Circulating CD49b+CD73+ B cells showed significantly increased frequency in patients with melanoma and eosinophilic esophagitis (EoE), two diseases associated with angiogenesis. In addition, tissue-infiltrating IgG4+CD49b+CD73+ B cells expressing proangiogenic cytokines were detected in patients with EoE and melanoma. Our results demonstrate a previously unidentified proangiogenic B cell subset characterized by expression of CD49b, CD73, and proangiogenic cytokines.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures

References
-
- van de Veen W., Stanic B., Wirz O. F., Jansen K., Globinska A., Akdis M., Role of regulatory B cells in immune tolerance to allergens and beyond. J. Allergy Clin. Immunol. 138, 654–665 (2016). - PubMed
-
- Harris D. P., Haynes L., Sayles P. C., Duso D. K., Eaton S. M., Lepak N. M., Johnson L. L., Swain S. L., Lund F. E., Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat. Immunol. 1, 475–482 (2000). - PubMed
-
- Bermejo D. A., Jackson S. W., Gorosito-Serran M., Acosta-Rodriguez E. V., Amezcua-Vesely M. C., Sather B. D., Singh A. K., Khim S., Mucci J., Liggitt D., Campetella O., Oukka M., Gruppi A., Rawlings D. J., Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORγt and Ahr that leads to IL-17 production by activated B cells. Nat. Immunol. 14, 514–522 (2013). - PMC - PubMed
-
- Jour G., Ivan D., Aung P. P., Angiogenesis in melanoma: An update with a focus on current targeted therapies. J. Clin. Pathol. 69, 472–483 (2016). - PubMed
-
- De Palma M., Biziato D., Petrova T. V., Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 17, 457–474 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

