Identification of distinct pathological signatures induced by patient-derived α-synuclein structures in nonhuman primates
- PMID: 32426502
- PMCID: PMC7220339
- DOI: 10.1126/sciadv.aaz9165
Identification of distinct pathological signatures induced by patient-derived α-synuclein structures in nonhuman primates
Abstract
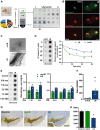
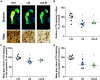
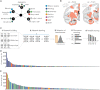
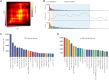
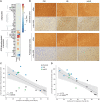
Dopaminergic neuronal cell death, associated with intracellular α-synuclein (α-syn)-rich protein aggregates [termed "Lewy bodies" (LBs)], is a well-established characteristic of Parkinson's disease (PD). Much evidence, accumulated from multiple experimental models, has suggested that α-syn plays a role in PD pathogenesis, not only as a trigger of pathology but also as a mediator of disease progression through pathological spreading. Here, we have used a machine learning-based approach to identify unique signatures of neurodegeneration in monkeys induced by distinct α-syn pathogenic structures derived from patients with PD. Unexpectedly, our results show that, in nonhuman primates, a small amount of singular α-syn aggregates is as toxic as larger amyloid fibrils present in the LBs, thus reinforcing the need for preclinical research in this species. Furthermore, our results provide evidence supporting the true multifactorial nature of PD, as multiple causes can induce a similar outcome regarding dopaminergic neurodegeneration.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures
References
-
- Braak H., Del Tredici K., Rüb U., de Vos R. A. I., Jansen Steur E. N. H., Braak E., Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24, 197–211 (2003). - PubMed
-
- Kordower J. H., Chu Y., Hauser R. A., Freeman T. B., Olanow C. W., Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14, 504–506 (2008). - PubMed
-
- Li J. Y., Englund E., Holton J. L., Soulet D., Hagell P., Lees A. J., Lashley T., Quinn N. P., Rehncrona S., Björklund A., Widner H., Revesz T., Lindvall O., Brundin P., Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 14, 501–503 (2008). - PubMed
-
- Dehay B., Vila M., Bezard E., Brundin P., Kordower J. H., Alpha-synuclein propagation: New insights from animal models. Mov. Disord. 31, 161–168 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

