The evolutionary origins of the cat attractant nepetalactone in catnip
- PMID: 32426505
- PMCID: PMC7220310
- DOI: 10.1126/sciadv.aba0721
The evolutionary origins of the cat attractant nepetalactone in catnip
Abstract
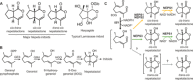
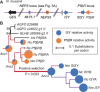
Catnip or catmint (Nepeta spp.) is a flowering plant in the mint family (Lamiaceae) famed for its ability to attract cats. This phenomenon is caused by the compound nepetalactone, a volatile iridoid that also repels insects. Iridoids are present in many Lamiaceae species but were lost in the ancestor of the Nepetoideae, the subfamily containing Nepeta. Using comparative genomics, ancestral sequence reconstructions, and phylogenetic analyses, we probed the re-emergence of iridoid biosynthesis in Nepeta. The results of these investigations revealed mechanisms for the loss and subsequent re-evolution of iridoid biosynthesis in the Nepeta lineage. We present evidence for a chronology of events that led to the formation of nepetalactone biosynthesis and its metabolic gene cluster. This study provides insights into the interplay between enzyme and genome evolution in the origins, loss, and re-emergence of plant chemical diversity.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures



Comment in
-
Tracking the Origin and Evolution of Plant Metabolites.Trends Plant Sci. 2020 Dec;25(12):1182-1184. doi: 10.1016/j.tplants.2020.08.010. Epub 2020 Sep 4. Trends Plant Sci. 2020. PMID: 32896488
References
-
- Tucker A. O., Tucker S. S., Catnip and the catnip response I. Econ. Bot. 42, 214–231 (1988).
-
- Bol S., Caspers J., Buckingham L., Anderson-Shelton G. D., Ridgway C., Buffington C. A., Schulz S., Bunnik E. M., Responsiveness of cats (Felidae) to silver vine (Actinidia polygama), Tatarian honeysuckle (Lonicera tatarica), valerian (Valeriana officinalis) and catnip (Nepeta cataria). BMC Vet. Res. 13, 70 (2017). - PMC - PubMed
-
- Eisner T., Catnip: Its raison d’etre. Science 146, 1318–1320 (1964). - PubMed
-
- Birkett M. A., Hassanali A., Hoglund S., Pettersson J., Pickett J. A., Repellent activity of catmint, Nepeta cataria, and iridoid nepetalactone isomers against Afro-tropical mosquitoes, ixodid ticks and red poultry mites. Phytochemistry 72, 109–114 (2011). - PubMed
-
- Miettinen K., Dong L., Navrot N., Schneider T., Burlat V., Pollier J., Woittiez L., van der Krol S., Lugan R., Ilc T., Verpoorte R., Oksman-Caldentey K.-M., Martinoia E., Bouwmeester H., Goossens A., Memelink J., Werck-Reichhart D., The seco-iridoid pathway from Catharanthus roseus. Nat. Commun. 5, 3606 (2014). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

