Heat-evolved microalgal symbionts increase coral bleaching tolerance
- PMID: 32426508
- PMCID: PMC7220355
- DOI: 10.1126/sciadv.aba2498
Heat-evolved microalgal symbionts increase coral bleaching tolerance
Abstract
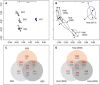
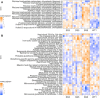
Coral reefs worldwide are suffering mass mortalities from marine heat waves. With the aim of enhancing coral bleaching tolerance, we evolved 10 clonal strains of a common coral microalgal endosymbiont at elevated temperatures (31°C) for 4 years in the laboratory. All 10 heat-evolved strains had expanded their thermal tolerance in vitro following laboratory evolution. After reintroduction into coral host larvae, 3 of the 10 heat-evolved endosymbionts also increased the holobionts' bleaching tolerance. Although lower levels of secreted reactive oxygen species (ROS) accompanied thermal tolerance of the heat-evolved algae, reduced ROS secretion alone did not predict thermal tolerance in symbiosis. The more tolerant symbiosis exhibited additional higher constitutive expression of algal carbon fixation genes and coral heat tolerance genes. These findings demonstrate that coral stock with enhanced climate resilience can be developed through ex hospite laboratory evolution of their microalgal endosymbionts.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works. Distributed under a Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
Figures


References
-
- Hughes T. P., Anderson K. D., Connolly S. R., Heron S. F., Kerry J. T., Lough J. M., Baird A. H., Baum J. K., Berumen M. L., Bridge T. C., Claar D. C., Eakin C. M., Gilmour J. P., Graham N. A. J., Harrison H., Hobbs J.-P. A., Hoey A. S., Hoogenboom M., Lowe R. J., McCulloch M. T., Pandolfi J. M., Pratchett M., Schoepf V., Torda G., Wilson S. K., Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018). - PubMed
-
- Hughes T. P., Kerry J. T., Baird A. H., Connolly S. R., Dietzel A., Eakin C. M., Heron S. F., Hoey A. S., Hoogenboom M. O., Liu G., McWilliam M. J., Pears R. J., Pratchett M. S., Skirving W. J., Stella J. S., Torda G., Global warming transforms coral reef assemblages. Nature 556, 492–496 (2018). - PubMed
-
- Hughes T. P., Kerry J. T., Connolly S. R., Baird A. H., Eakin C. M., Heron S. F., Hoey A. S., Hoogenboom M. O., Jacobson M., Liu G., Pratchett M. S., Skirving W., Torda G., Ecological memory modifies the cumulative impact of recurrent climate extremes. Nat. Clim. Chang. 9, 40–43 (2019).
-
- Hughes T. P., Kerry J. T., Baird A. H., Connolly S. R., Chase T. J., Dietzel A., Hill T., Hoey A. S., Hoogenboom M. O., Jacobson M., Kerswell A., Madin J. S., Mieog A., Paley A. S., Pratchett M. S., Torda G., Woods R. M., Global warming impairs stock–recruitment dynamics of corals. Nature 568, 387–390 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

