Inhibitory effect of red LED irradiation on fibroblasts and co-culture of adipose-derived mesenchymal stem cells
- PMID: 32426535
- PMCID: PMC7226671
- DOI: 10.1016/j.heliyon.2020.e03882
Inhibitory effect of red LED irradiation on fibroblasts and co-culture of adipose-derived mesenchymal stem cells
Abstract
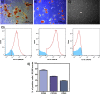
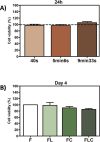
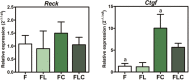
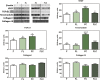
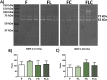
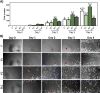
The objective of this study was to evaluate the effects of red Light Emiting Diode (red LED) irradiation on fibroblasts in adipose-derived mesenchymal stem cells (ASC) co-culture on the scratch assay. We hypothesized that red LED irradiation could stimulate paracrine secretion of ASC, contributing to the activation of genes and molecules involved in cell migration and tissue repair. ASC were co-cultured with NIH/3T3 fibroblasts through direct contact and subjected to red LED irradiation (1.45 J/cm2/5min6s) after the scratch assay, during 4 days. Four groups were established: fibroblasts (F), fibroblasts + LED (FL), fibroblasts + ASC (FC) and fibroblasts + LED + ASC (FLC). The analyzes were based on Ctgf and Reck expression, quantification of collagen types I and III, tenomodulin, VEGF, TGF-β1, MMP-2 and MMP-9, as well as viability analysis and cell migration. Higher Ctgf expression was observed in FC compared to F. Group FC presented higher amount of tenomodulin and VEGF in relation to the other groups. In the cell migration analysis, a higher number of cells was observed in the scratched area of the FC group on the 4th day. There were no differences between groups considering cell viability, Reck expression, amount of collagen types I and III, MMP-2 and TGF-β1, whereas TGF-β1 was not detected in the FC group and the MMP-9 in none of the groups. Our hypothesis was not supported by the results because the red LED irradiation decreased the healing response of ASC. An inhibitory effect of the LED irradiation associated with ASC co-culture was observed with reduction of the amount of TGF-β1, VEGF and tenomodulin, possibly involved in the reduced cell migration. In turn, the ASC alone seem to have modulated fibroblast behavior by increasing Ctgf, VEGF and tenomodulin, leading to greater cell migration. In conclusion, red LED and ASC therapy can have independent effects on fibroblast wound healing, but the combination of both does not have a synergistic effect. Therefore, future studies with other parameters of red LED associated with ASC should be tested aiming clinical application for tissue repair.
Keywords: Biological sciences; Biomedical engineering; Cell biology; Cell migration; Ctgf; Molecular biology; Proteins; Regenerative medicine; Repair; Scratch assay; TGF-β1; Tenomodulin.
© 2020 Published by Elsevier Ltd.
Figures
References
-
- Aro A.A., Nishan U., Perez M.O., Rodrigues R.A., Foglio M.A., Carvalho J.E., Gomes L., Vidal B.C., Pimentel E.R. Structural and biochemical alterations during the healing process of tendons treated with Aloe vera. Life Sci. 2012;91(17-18):885–893. - PubMed
-
- Bacakova L., Zarubova J., Travnickova M., Musilkova J., Pajorova J., Slepicka P., Kasalkova N.S., Svorcik V., Kolska Z., Motarjemi H., Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol. Adv. 2018;36(4):1111–1126. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

