Incomplete data analysis of non-inferiority clinical trials: Difference between binomial proportions case
- PMID: 32426549
- PMCID: PMC7226649
- DOI: 10.1016/j.conctc.2020.100567
Incomplete data analysis of non-inferiority clinical trials: Difference between binomial proportions case
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.Contemp Clin Trials Commun. 2020 Dec 10;20:100691. doi: 10.1016/j.conctc.2020.100691. eCollection 2020 Dec. Contemp Clin Trials Commun. 2020. PMID: 33392415 Free PMC article.
Abstract
Background: Incomplete data analysis continues to be a major issue for non-inferiority clinical trials. Due to the steadily increasing use of non-inferiority study design, we believe this topic deserves an immediate attention.
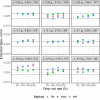
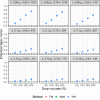
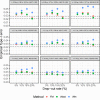
Methods: We evaluated the performance of various strategies, including complete case analysis and various imputations techniques for handling incomplete non-inferiority clinical trials when outcome of interest is difference between binomial proportions. Non-inferiority of a new treatment was determined using a fixed margin approach with 95-95% confidence interval method. The methods used to construct the confidence intervals were compared as well and included: Wald, Farrington-Manning and Newcombe methods.
Results: We found that worst-case and best-case scenario imputation methods should not be used for analysis of incomplete data in non-inferiority trial design, since such methods seriously inflate type-I error rates and produce biased estimates. In addition, we report conditions under which complete case analysis is an acceptable strategy for missing at random missingness mechanism. Importantly, we show how two-stage multiple imputation could be successfully applied for incomplete data that follow missing not at random patterns, and thus result in controlled type-I error rates and unbiased estimates.
Conclusion: This thorough simulation study provides a road map for the analysis of incomplete data in non-inferiority clinical trials for different types of missingness. We believe that the results reported in this paper could serve practitioners who encounter missing data problems in their non-inferiority clinical trials.
Keywords: Binary outcome; Incomplete data analysis; Multiple imputation; Non-inferiority design.
© 2020 The Authors.
Figures

References
-
- FDA . 2016. Non-inferiority Clinical Trials to Establish Effectiveness; Guidance for Industry.
-
- Piaggio G., Elbourne D.R., Pocock S.J., Evans S.J., Altman D.G., Group C. Reporting of noninferiority and equivalence randomized trials: extension of the consort 2010 statement. Jama. 2012;308(24):2594–2604. - PubMed
-
- ICH . 2000. Choice of control group and related issues in clinical trials e10. - PubMed
-
- Little R.J., Rubin D.B. vol. 333. John Wiley & Sons; 2014. (Statistical Analysis with Missing Data).
-
- Collins L.M., Schafer J.L., Kam C.-M. A comparison of inclusive and restrictive strategies in modern missing data procedures. Psychol. Methods. 2001;6(4):330. - PubMed
LinkOut - more resources
Full Text Sources

