Molecular Recognition in C-Type Lectins: The Cases of DC-SIGN, Langerin, MGL, and L-Sectin
- PMID: 32426893
- PMCID: PMC7276794
- DOI: 10.1002/cbic.202000238
Molecular Recognition in C-Type Lectins: The Cases of DC-SIGN, Langerin, MGL, and L-Sectin
Abstract
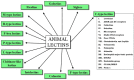
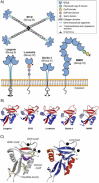
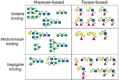
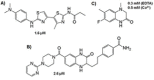
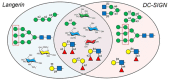
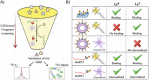
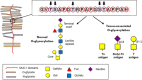
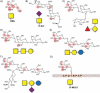
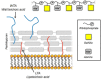
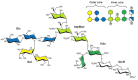
Carbohydrates play a pivotal role in intercellular communication processes. In particular, glycan antigens are key for sustaining homeostasis, helping leukocytes to distinguish damaged tissues and invading pathogens from healthy tissues. From a structural perspective, this cross-talk is fairly complex, and multiple membrane proteins guide these recognition processes, including lectins and Toll-like receptors. Since the beginning of this century, lectins have become potential targets for therapeutics for controlling and/or avoiding the progression of pathologies derived from an incorrect immune outcome, including infectious processes, cancer, or autoimmune diseases. Therefore, a detailed knowledge of these receptors is mandatory for the development of specific treatments. In this review, we summarize the current knowledge about four key C-type lectins whose importance has been steadily growing in recent years, focusing in particular on how glycan recognition takes place at the molecular level, but also looking at recent progresses in the quest for therapeutics.
Keywords: drug discovery; glycans; lectins; molecular recognition.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

