Protein quality in ready-to-use supplementary foods for moderate wasting
- PMID: 32426949
- PMCID: PMC7507576
- DOI: 10.1111/mcn.13019
Protein quality in ready-to-use supplementary foods for moderate wasting
Abstract
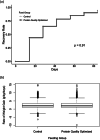
There are no guidelines for the optimal protein quality of ready-to-supplementary food (RUSF) for moderate acute malnutrition (MAM). This randomized, controlled, double-blinded, clinical effectiveness trial evaluated two RUSFs in the treatment of MAM. Both foods contained greater than 7% dairy protein, but the protein-optimized RUSF had a calculated digestible indispensable amino acid score (DIAAS) of 95%, whereas the control RUSF had a calculated DIAAS of 63%. There were 1,737 rural Malawian children 6-59 months of age treated with 75 kcal/kg/day of either control or protein quality-optimized RUSF for up to 12 weeks. There was no difference in the proportion of children who recovered from MAM between the group that received protein-optimized RUSF (759/860, 88%) and the group that received control RUSF (766/877, 87%, difference 1%, 95% CI, -2.1 to 4.1, p = 0.61). There were no differences in time to recovery or average weight gain; nor were adverse effects reported. Both RUSFs showed indistinguishable clinical outcomes, with recovery rates higher than typically seen in treatment for MAM. The DIAAS of these two RUSFs was measured using a pig model. Unexpectedly, the protein quality of the optimized RUSF was inferior to the control RUSF: DIAAS = 82% for the protein quality optimized RUSF and 96% for control RUSF. The controlled conditions of this trial suggest that in supplementary food products for MAM, protein quality is not an independent predictor of clinical effectiveness.
Keywords: Malawi; dairy products; indispensable amino acids; moderate acute malnutrition; protein quality; ready-to-use supplementary food; wasting.
© 2020 The Authors. Maternal & Child Nutrition published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
-
- Blok, M. C. , & Spek, J. W. (2016). CVB Feed Table 2016. Retrieved from www.cvbdiervoeding.nl
-
- Briend, A. , Akomo, P. , Bahwere, P. , De Pee, S. , Dibari, F. , Golden, M. H. , … Ryan, K. (2015). Developing food supplements for moderately malnourished children: Lessons learned from ready‐to‐use therapeutic foods. Food and Nutrition Bulletin, 36(1), S53–S58. 10.1177/15648265150361S109 - DOI - PubMed
-
- Coates, J. , Swindale, A. , & Bilinsky, P. (2007). USAID Food and Nutrition Technical Assistance: Household Food Insecurity Access Scale (HFIAS) for measurement of food access: Indicator guide. Organization, (August).
-
- Eaton, J. C. , Rothpletz‐Puglia, P. , Dreker, M. R. , Iannotti, L. , Lutter, C. , Kaganda, J. , & Rayco‐Solon, P. (2019). Effectiveness of provision of animal‐source foods for supporting optimal growth and development in children 6 to 59 months of age. The Cochrane Database of Systematic Reviews, 2(2), CD012818 10.1002/14651858.CD012818.pub2 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

