Ten-Year Trends in Enrollment of Women and Minorities in Pivotal Trials Supporting Recent US Food and Drug Administration Approval of Novel Cardiometabolic Drugs
- PMID: 32427023
- PMCID: PMC7428976
- DOI: 10.1161/JAHA.119.015594
Ten-Year Trends in Enrollment of Women and Minorities in Pivotal Trials Supporting Recent US Food and Drug Administration Approval of Novel Cardiometabolic Drugs
Abstract
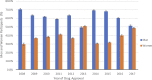
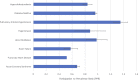
Background In 1993, the US Food and Drug Administration established guidelines to increase diversity by sex and race/ethnicity of participants in clinical trials supporting novel drug approvals. In this study we investigated the 10-year trends of participation of women and minorities in pivotal trials supporting approval of new molecular entities in cardiometabolic drugs from January 2008 to December 2017. Methods and Results A list of new molecular entities was abstracted from publicly available data at Drugs@Fda. Sex and race/ethnicity data were collected from trial publications. Linear regression analysis was performed to assess the relation between drug approval year and proportion of women and minorities enrolled. Thirty-five novel cardiovascular (n=24) and diabetes mellitus (n=11) drugs were approved by the US Food and Drug Administration during the study period. The median number of participants supporting each drug was 5930 (interquartile range, 3175-10 942). Women represented 36% (n=108 052) of trial participants (n=296 163). Women were underrepresented compared with their proportion of the disease population in trials of coronary heart disease (participation-to-prevalence ratio, 0.52), heart failure (participation-to-prevalence ratio, 0.58), and acute coronary syndrome (participation-to-prevalence ratio, 0.68). Among trial participants, 81% were white, 4% black, 12% Asian, and 11% Hispanic/Latino. There was no significant association between enrollment of women (P=0.29) or underrepresented minorities (P=0.45) with the drug approval year. Conclusions Over the past decade (2008-2017), women and minorities, particularly blacks, have continued to be inadequately represented in pivotal cardiometabolic clinical trials that support US Food and Drug Administration approval of new molecular entities. This may have major implications in determining efficacy of such therapies in these groups, and may impair generalizability of trial results to routine clinical practice.
Keywords: cardiometabolic drugs; clinical trials; minorities; women.
Figures

References
-
- Virani S, Alonso A, Benjamin E, Bittencourt M, Callaway C, Carson A, Chamberlain A, Chang A, Cheng S, Delling F, et al. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596. - PubMed
-
- Centers for Disease Control and Prevention . Diabetes and women. 2018. Available at: https://www.cdc.gov/features/diabetes-women/index.html. Accessed April 15, 2020.
-
- US General Accounting Office . Women's Health: FDA needs to ensure more study of gender differences in prescription drug testing. 1992. Available at: http://archive.gao.gov/d35t11/147861.pdf. Accessed April 15, 2020.
-
- Meinert CL, Gilpin AK, Unalp A, Dawson C. Gender representation in trials. Control Clin Trials. 2000;21:462–475. - PubMed
-
- Fadiran EO, Zhang L. Effects of Sex Differences in the Pharmacokinetics of Drugs and Their Impact on the Safety of Medicines in Women. Medicines for Women. Switzerland: Springer International Publishing; 2015:41–68.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

