Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19
- PMID: 32427279
- PMCID: PMC7314133
- DOI: 10.1093/cid/ciaa601
Early Short-Course Corticosteroids in Hospitalized Patients With COVID-19
Abstract
Background: There is no proven antiviral or immunomodulatory therapy for coronavirus disease 2019 (COVID-19). The disease progression associated with the proinflammatory host response prompted us to examine the role of early corticosteroid therapy in patients with moderate to severe COVID-19.
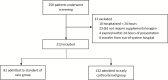
Methods: We conducted a single pretest, single posttest quasi-experiment in a multicenter health system in Michigan from 12 March to 27 March 2020. Adult patients with confirmed moderate to severe COVID were included. A protocol was implemented on 20 March 2020 using early, short-course, methylprednisolone 0.5 to 1 mg/kg/day divided in 2 intravenous doses for 3 days. Outcomes of standard of care (SOC) and early corticosteroid groups were evaluated, with a primary composite endpoint of escalation of care from ward to intensive care unit (ICU), new requirement for mechanical ventilation, and mortality. All patients had at least 14 days of follow-up.
Results: We analyzed 213 eligible subjects, 81 (38%) and 132 (62%) in SOC and early corticosteroid groups, respectively. The composite endpoint occurred at a significantly lower rate in the early corticosteroid group (34.9% vs 54.3%, P = .005). This treatment effect was observed within each individual component of the composite endpoint. Significant reduction in median hospital length of stay was also observed in the early corticosteroid group (5 vs 8 days, P < .001). Multivariate regression analysis demonstrated an independent reduction in the composite endpoint at 14-days controlling for other factors (adjusted odds ratio: 0.41; 95% confidence interval, .22 - .77).
Conclusions: An early short course of methylprednisolone in patients with moderate to severe COVID-19 reduced escalation of care and improved clinical outcomes.
Clinical trials registration: NCT04374071.
Keywords: COVID-19; Corticosteroids; SARS-COV-2; coronavirus; outcomes.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
The Right Time for Steroids in COVID-19.Clin Infect Dis. 2021 Apr 26;72(8):1486-1487. doi: 10.1093/cid/ciaa865. Clin Infect Dis. 2021. PMID: 32585016 Free PMC article. No abstract available.
-
Reply to Fernandez Cruz, et al.Clin Infect Dis. 2021 Apr 26;72(8):1487. doi: 10.1093/cid/ciaa870. Clin Infect Dis. 2021. PMID: 32589700 No abstract available.
References
-
- Centers for Disease Control and Prevention. Coronavirus disease 2019 (COVID-19): cases and latest updates. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Accessed 7 April 2020
-
- Centers for Disease Control and Prevention. COVID-NET: COVID-19 associated hospitalization surveillance network. Available at: https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html. Accessed 8 April 2020.
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous