Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019
- PMID: 32427334
- PMCID: PMC7313936
- DOI: 10.1093/infdis/jiaa273
Sensitivity in Detection of Antibodies to Nucleocapsid and Spike Proteins of Severe Acute Respiratory Syndrome Coronavirus 2 in Patients With Coronavirus Disease 2019
Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with respiratory-related disease and death. Assays to detect virus-specific antibodies are important to understand the prevalence of infection and the course of the immune response.
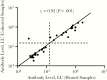
Methods: Quantitative measurements of plasma or serum antibodies to the nucleocapsid and spike proteins were analyzed using luciferase immunoprecipitation system assays in 100 cross-sectional or longitudinal samples from patients with SARS-CoV-2 infection. A subset of samples was tested both with and without heat inactivation.
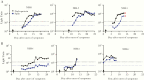
Results: At >14 days after symptom onset, antibodies against SARS-CoV-2 nucleocapsid protein showed 100% sensitivity and 100% specificity, whereas antibodies to spike protein were detected with 91% sensitivity and 100% specificity. Neither antibody levels nor the rate of seropositivity were significantly reduced by heat inactivation of samples. Analysis of daily samples from 6 patients with COVID-19 showed anti-nucleocapsid and spike protein antibodies appearing between days 8 and 14 after initial symptoms. Immunocompromised patients generally had a delayed antibody response to SARS-CoV-2, compared with immunocompetent patients.
Conclusions: Antibody to the nucleocapsid protein of SARS-CoV-2 is more sensitive than spike protein antibody for detecting early infection. Analyzing heat-inactivated samples with a luciferase immunoprecipitation system assay is a safe and sensitive method for detecting SARS-CoV-2 antibodies.
Keywords: COVID-19; SARS-CoV-2; coronavirus; serology.
Published by Oxford University Press for the Infectious Diseases Society of America 2020.
Figures

Update of
-
Detection of Nucleocapsid Antibody to SARS-CoV-2 is More Sensitive than Antibody to Spike Protein in COVID-19 Patients.medRxiv [Preprint]. 2020 Apr 24:2020.04.20.20071423. doi: 10.1101/2020.04.20.20071423. medRxiv. 2020. Update in: J Infect Dis. 2020 Jun 29;222(2):206-213. doi: 10.1093/infdis/jiaa273. PMID: 32511445 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

