Influence of Storage Conditions on SARS-CoV-2 Nucleic Acid Detection in Throat Swabs
- PMID: 32427340
- PMCID: PMC7313924
- DOI: 10.1093/infdis/jiaa272
Influence of Storage Conditions on SARS-CoV-2 Nucleic Acid Detection in Throat Swabs
Abstract
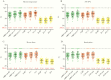
The detection of SARS-CoV-2 infection is the premise of quarantine. In many countries or areas, samples need to be shipped or inactivated before SARS-CoV-2 testing. In this study, we checked the influence of sample storage conditions on SARS-CoV-2 nucleic acid testing results, including sample inactivation time, storage temperature, and storage time. All of these conditions caused an increase in the cycle threshold values of the nucleic acid tests and led to the misclassification of at least 10.2% of positive cases as negative or suspected. The results highlight the importance of immediate testing of samples for SARS-CoV-2 nucleic acid detection.
Keywords: influence; SARS-CoV-2; nucleic acid detection; storage; throat swab.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures
References
-
- World Health Organization. Coronavirus disease 2019 (COVID-19) situation report-69. https://www.who.int/docs/default-source/coronaviruse/situation-reports/2.... Accessed 26 April 2020.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

