A novel 3',5'-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCδ/JNK signal in prostate cancer
- PMID: 32427575
- PMCID: PMC7288973
- DOI: 10.18632/aging.103178
A novel 3',5'-diprenylated chalcone induces concurrent apoptosis and GSDME-dependent pyroptosis through activating PKCδ/JNK signal in prostate cancer
Abstract
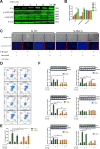
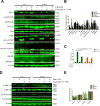
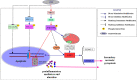
Although androgen deprivation therapy may initially be effective in prostate cancer, the disease can gradually progress to castration-resistant prostate cancer, at which point chemotherapy becomes the major clinical strategy. In this study, we demonstrated the anti-cancer potential of a novel 3',5'-diprenylated chalcone (C10), which selectively inhibited the proliferation of PC3 cells in vitro and in vivo. C10 treatment elevated the proportion of PC3 cells in sub-G1 phase and induced programmed cell death. Interestingly, C10 elicited concurrent Caspase-dependent apoptotic and gasdermin E-dependent pyroptotic events. RNA-Seq and bioinformatics analyses revealed a strong correlation between protein kinase C delta (PKCδ) and mitogen-activated protein kinase pathway activation in prostate cancer. PKCδ silencing in PC3 cells suppressed the activation of the JNK pathway and the expression of its downstream genes, including Bax, interleukin-6 and interleukin-1β, which are involved in apoptotic and pyroptotic processes. Moreover, in PC3 cell xenograft tumor tissues, C10 treatment inhibited tumor growth and upregulated PKCδ. These findings suggest that C10 treatment induces the PKCδ/JNK pathway, thereby activating Caspase-3 and inducing the cleavage of PARP and gasdermin E to execute apoptosis and cell-lytic pyroptosis in prostate cancer cells.
Keywords: 3’,5’-diprenylated chalcone; PKCδ; apoptosis; crosstalk; pyroptosis.
Conflict of interest statement
Figures






References
-
- Tucci M, Caffo O, Buttigliero C, Cavaliere C, D’aniello C, Di Maio M, Kinspergher S, Maines F, Rizzo M, Rossetti S, Veccia A, Scagliotti GV, Facchini G. Therapeutic options for first-line metastatic castration-resistant prostate cancer: suggestions for clinical practise in the CHAARTED and LATITUDE era. Cancer Treat Rev. 2019; 74:35–42. 10.1016/j.ctrv.2019.01.002 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

