Selective role of Nck1 in atherogenic inflammation and plaque formation
- PMID: 32427580
- PMCID: PMC8011212
- DOI: 10.1172/JCI135552
Selective role of Nck1 in atherogenic inflammation and plaque formation
Abstract
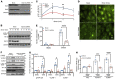
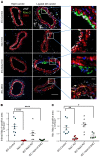
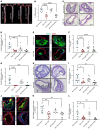
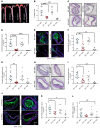
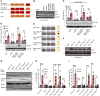
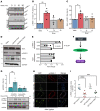
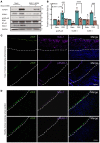
Although the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS) established the role of treating inflammation in atherosclerosis, our understanding of endothelial activation at atherosclerosis-prone sites remains limited. Disturbed flow at atheroprone regions primes plaque inflammation by enhancing endothelial NF-κB signaling. Herein, we demonstrate a role for the Nck adaptor proteins in disturbed flow-induced endothelial activation. Although highly similar, only Nck1 deletion, but not Nck2 deletion, limited flow-induced NF-κB activation and proinflammatory gene expression. Nck1-knockout mice showed reduced endothelial activation and inflammation in both models, disturbed flow- and high fat diet-induced atherosclerosis, whereas Nck2 deletion did not. Bone marrow chimeras confirmed that vascular Nck1, but not hematopoietic Nck1, mediated this effect. Domain-swap experiments and point mutations identified the Nck1 SH2 domain and the first SH3 domain as critical for flow-induced endothelial activation. We further characterized Nck1's proinflammatory role by identifying interleukin 1 type I receptor kinase-1 (IRAK-1) as a Nck1-selective binding partner, demonstrating that IRAK-1 activation by disturbed flow required Nck1 in vitro and in vivo, showing endothelial Nck1 and IRAK-1 staining in early human atherosclerosis, and demonstrating that disturbed flow-induced endothelial activation required IRAK-1. Taken together, our data reveal a hitherto unknown link between Nck1 and IRAK-1 in atherogenic inflammation.
Keywords: Atherosclerosis; Cell Biology; Signal transduction; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures


Comment in
-
Nck1 is a critical adaptor between proatherogenic blood flow, inflammation, and atherosclerosis.J Clin Invest. 2020 Aug 3;130(8):3968-3970. doi: 10.1172/JCI138536. J Clin Invest. 2020. PMID: 32657777 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

