Clinical and pathological investigation of patients with severe COVID-19
- PMID: 32427582
- PMCID: PMC7406259
- DOI: 10.1172/jci.insight.138070
Clinical and pathological investigation of patients with severe COVID-19
Abstract
Background: Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory coronavirus 2 (SARS-CoV-2), has become a pandemic. This study addresses the clinical and immunopathological characteristics of severe COVID-19.
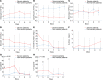
Methods: Sixty-nine patients with COVID-19 were classified into severe and nonsevere groups to analyze their clinical and laboratory characteristics. A panel of blood cytokines was quantified over time. Biopsy specimens from 2 deceased cases were obtained for immunopathological, ultrastructural, and in situ hybridization examinations.
Results: Circulating cytokines, including IL-8, IL-6, TNF-α, IP10, MCP1, and RANTES, were significantly elevated in patients with severe COVID-19. Dynamic IL-6 and IL-8 were associated with disease progression. SARS-CoV-2 was demonstrated to infect type II and type I pneumocytes and endothelial cells, leading to severe lung damage through cell pyroptosis and apoptosis. In severe cases, lymphopenia, neutrophilia, depletion of CD4+ and CD8+ T lymphocytes, and massive macrophage and neutrophil infiltrates were observed in both blood and lung tissues.
Conclusions: A panel of circulating cytokines could be used to predict disease deterioration and inform clinical interventions. Severe pulmonary damage was predominantly attributed to both cytopathy caused by SARS-CoV-2 and immunopathologic damage. Strategies that prohibit pulmonary recruitment and overactivation of inflammatory cells by suppressing cytokine storm might improve the outcomes of patients with severe COVID-19.
Keywords: Apoptosis; COVID-19; Infectious disease; Macrophages; Medical statistics; Pulmonology.
Conflict of interest statement
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

