TrkB agonists prevent postischemic emergence of refractory neonatal seizures in mice
- PMID: 32427585
- PMCID: PMC7406252
- DOI: 10.1172/jci.insight.136007
TrkB agonists prevent postischemic emergence of refractory neonatal seizures in mice
Abstract
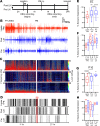
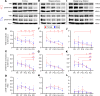
Refractory neonatal seizures do not respond to first-line antiseizure medications like phenobarbital (PB), a positive allosteric modulator for GABAA receptors. GABAA receptor-mediated inhibition is dependent upon electroneutral cation-chloride transporter KCC2, which mediates neuronal chloride extrusion and its age-dependent increase and postnatally shifts GABAergic signaling from depolarizing to hyperpolarizing. Brain-derived neurotropic factor-tyrosine receptor kinase B activation (BDNF-TrkB activation) after excitotoxic injury recruits downstream targets like PLCγ1, leading to KCC2 hypofunction. Here, the antiseizure efficacy of TrkB agonists LM22A-4, HIOC, and deoxygedunin (DG) on PB-refractory seizures and postischemic TrkB pathway activation was investigated in a mouse model (CD-1, P7) of refractory neonatal seizures. LM, a BDNF loop II mimetic, rescued PB-refractory seizures in a sexually dimorphic manner. Efficacy was associated with a substantial reduction in the postischemic phosphorylation of TrkB at Y816, a site known to mediate postischemic KCC2 hypofunction via PLCγ1 activation. LM rescued ischemia-induced phospho-KCC2-S940 dephosphorylation, preserving its membrane stability. Full TrkB agonists HIOC and DG similarly rescued PB refractoriness. Chemogenetic inactivation of TrkB substantially reduced postischemic neonatal seizure burdens at P7. Sex differences identified in developmental expression profiles of TrkB and KCC2 may underlie the sexually dimorphic efficacy of LM. These results support a potentially novel role for the TrkB receptor in the emergence of age-dependent refractory neonatal seizures.
Keywords: Development; Neuroscience; Seizures.
Conflict of interest statement
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

