Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke
- PMID: 32427863
- PMCID: PMC7237502
- DOI: 10.1038/s41467-020-16191-y
Neutrophil extracellular traps released by neutrophils impair revascularization and vascular remodeling after stroke
Abstract
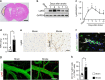
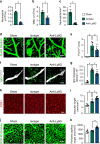
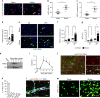
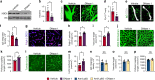
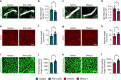
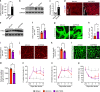
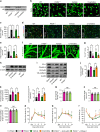
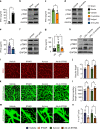
Neovascularization and vascular remodeling are functionally important for brain repair after stroke. We show that neutrophils accumulate in the peri-infarct cortex during all stages of ischemic stroke. Neutrophils producing intravascular and intraparenchymal neutrophil extracellular traps (NETs) peak at 3-5 days. Neutrophil depletion reduces blood-brain barrier (BBB) breakdown and enhances neovascularization at 14 days. Peptidylarginine deiminase 4 (PAD4), an enzyme essential for NET formation, is upregulated in peri-ischemic brains. Overexpression of PAD4 induces an increase in NET formation that is accompanied by reduced neovascularization and increased BBB damage. Disruption of NETs by DNase 1 and inhibition of NET formation by genetic ablation or pharmacologic inhibition of PAD increases neovascularization and vascular repair and improves functional recovery. Furthermore, PAD inhibition reduces stroke-induced STING-mediated production of IFN-β, and STING knockdown and IFN receptor-neutralizing antibody treatment reduces BBB breakdown and increases vascular plasticity. Collectively, our results indicate that NET release impairs vascular remodeling during stroke recovery.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Neutrophil extracellular traps promote tPA-induced brain hemorrhage via cGAS in mice with stroke.Blood. 2021 Jul 8;138(1):91-103. doi: 10.1182/blood.2020008913. Blood. 2021. PMID: 33881503 Free PMC article.
-
PAD4 Deficiency Improves Bleomycin-induced Neutrophil Extracellular Traps and Fibrosis in Mouse Lung.Am J Respir Cell Mol Biol. 2020 Dec;63(6):806-818. doi: 10.1165/rcmb.2019-0433OC. Am J Respir Cell Mol Biol. 2020. PMID: 32915635
-
Neutrophil Targeting Platform Reduces Neutrophil Extracellular Traps for Improved Traumatic Brain Injury and Stroke Theranostics.Adv Sci (Weinh). 2024 Jun;11(21):e2308719. doi: 10.1002/advs.202308719. Epub 2024 Mar 23. Adv Sci (Weinh). 2024. PMID: 38520727 Free PMC article.
-
Neutrophil Extracellular Traps Exacerbate Ischemic Brain Damage.Mol Neurobiol. 2022 Jan;59(1):643-656. doi: 10.1007/s12035-021-02635-z. Epub 2021 Nov 8. Mol Neurobiol. 2022. PMID: 34748205 Review.
-
Inhibition of Neutrophil Extracellular Traps: A Potential Therapeutic Strategy for Hemorrhagic Stroke.J Integr Neurosci. 2025 Apr 23;24(4):26357. doi: 10.31083/JIN26357. J Integr Neurosci. 2025. PMID: 40302254 Review.
Cited by
-
A case of ischemic stroke with hemorrhagic transformation associated with essential thrombocythemia and JAK-2 V617F mutation.BMC Neurol. 2022 Nov 17;22(1):437. doi: 10.1186/s12883-022-02964-z. BMC Neurol. 2022. PMID: 36397023 Free PMC article.
-
Nanotechnology approaches to drug delivery for the treatment of ischemic stroke.Bioact Mater. 2024 Sep 23;43:145-161. doi: 10.1016/j.bioactmat.2024.09.016. eCollection 2025 Jan. Bioact Mater. 2024. PMID: 39386225 Free PMC article. Review.
-
The correlation between novel peripheral blood cell ratios and 90-day mortality in patients with acute ischemic stroke.PLoS One. 2020 Aug 28;15(8):e0238312. doi: 10.1371/journal.pone.0238312. eCollection 2020. PLoS One. 2020. PMID: 32857820 Free PMC article.
-
DNMT3A dysfunction promotes neuroinflammation and exacerbates acute ischemic stroke.MedComm (2020). 2024 Jul 14;5(7):e652. doi: 10.1002/mco2.652. eCollection 2024 Jul. MedComm (2020). 2024. PMID: 39006763 Free PMC article.
-
The role of P-selectin/PSGL-1 in regulating NETs as a novel mechanism in cerebral ischemic injury.Front Neurol. 2024 Jul 3;15:1442613. doi: 10.3389/fneur.2024.1442613. eCollection 2024. Front Neurol. 2024. PMID: 39022737 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

