Induction of posterior vitreous detachment (PVD) by non-enzymatic reagents targeting vitreous collagen liquefaction as well as vitreoretinal adhesion
- PMID: 32427865
- PMCID: PMC7237681
- DOI: 10.1038/s41598-020-64931-3
Induction of posterior vitreous detachment (PVD) by non-enzymatic reagents targeting vitreous collagen liquefaction as well as vitreoretinal adhesion
Erratum in
-
Author Correction: Induction of posterior vitreous detachment (PVD) by non-enzymatic reagents targeting vitreous collagen liquefaction as well as vitreoretinal adhesion.Sci Rep. 2020 Jul 16;10(1):12083. doi: 10.1038/s41598-020-69093-w. Sci Rep. 2020. PMID: 32669590 Free PMC article.
Abstract
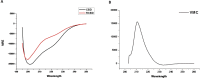
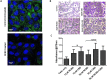
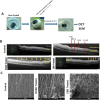
Induction of posterior vitreous detachment (PVD) by pharmacologic vitreolysis has been largely attempted through the use of enzymatic reagents. Ocriplasmin has been the only FDA-approved clinical reagent so far. Several adverse effects of ocriplasmin have emerged, however, and the search for alternative PVD-inducing reagents continues. Since i) collagen forms an important structural component of the vitreous, and ii) strong vitreo-retinal adhesions exist between the cortical vitreous and the internal limiting membrane (ILM) of the retina, an effective PVD-inducing reagent would require both, vitreous liquefaction, and concurrent dehiscence of vitreoretinal adhesion, without being toxic to retinal cells. We designed a combination of two reagents to achieve these two objectives; a triple helix-destabilizing collagen binding domain (CBD), and a fusion of RGD (integrin-binding) tripeptide with CBD (RCBD) to facilitate separation of posterior cortical vitreous from retinal surface. Based on in vitro, ex-vivo, and in vivo experiments, we show that a combination of CBD and RCBD displays potential for safe pharmacologic vitreolysis. Our findings assume significance in light of the fact that synthetic RGD-containing peptides have already been used for inhibition of tumor cell invasion. Proteins such as variants of collagen binding domains could have extended therapeutic uses in the future.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Lund-Andersen H, Sebag J, Sander B, La Cour M. The vitreous. Advances in Organ Biology. 2005;10:181–194. doi: 10.1016/S1569-2590(05)10007-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

