Establishment of an in vitro RNA polymerase transcription system: a new tool to study transcriptional activation in Borrelia burgdorferi
- PMID: 32427963
- PMCID: PMC7237435
- DOI: 10.1038/s41598-020-65104-y
Establishment of an in vitro RNA polymerase transcription system: a new tool to study transcriptional activation in Borrelia burgdorferi
Abstract
The Lyme disease spirochete Borrelia burgdorferi exhibits dramatic changes in gene expression as it transits between its tick vector and vertebrate host. A major hurdle to understanding the mechanisms underlying gene regulation in B. burgdorferi has been the lack of a functional assay to test how gene regulatory proteins and sigma factors interact with RNA polymerase to direct transcription. To gain mechanistic insight into transcriptional control in B. burgdorferi, and address sigma factor function and specificity, we developed an in vitro transcription assay using the B. burgdorferi RNA polymerase holoenzyme. We established reaction conditions for maximal RNA polymerase activity by optimizing pH, temperature, and the requirement for divalent metals. Using this assay system, we analyzed the promoter specificity of the housekeeping sigma factor RpoD to promoters encoding previously identified RpoD consensus sequences in B. burgdorferi. Collectively, this study established an in vitro transcription assay that revealed RpoD-dependent promoter selectivity by RNA polymerase and the requirement of specific metal cofactors for maximal RNA polymerase activity. The establishment of this functional assay will facilitate molecular and biochemical studies on how gene regulatory proteins and sigma factors exert control of gene expression in B. burgdorferi required for the completion of its enzootic cycle.
Conflict of interest statement
The authors declare no competing interests.
Figures

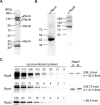

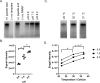

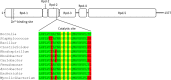

Similar articles
-
Two Distinct Mechanisms Govern RpoS-Mediated Repression of Tick-Phase Genes during Mammalian Host Adaptation by Borrelia burgdorferi, the Lyme Disease Spirochete.mBio. 2017 Aug 22;8(4):e01204-17. doi: 10.1128/mBio.01204-17. mBio. 2017. PMID: 28830947 Free PMC article.
-
Essential Components of Borreliella (Borrelia) burgdorferi In Vitro Transcription Assays.J Vis Exp. 2022 Jul 22;(185). doi: 10.3791/64134. J Vis Exp. 2022. PMID: 35938842
-
Analysis of promoter elements involved in the transcriptional initiation of RpoS-dependent Borrelia burgdorferi genes.J Bacteriol. 2004 Nov;186(21):7390-402. doi: 10.1128/JB.186.21.7390-7402.2004. J Bacteriol. 2004. PMID: 15489451 Free PMC article.
-
Gene regulation in Borrelia burgdorferi.Annu Rev Microbiol. 2011;65:479-99. doi: 10.1146/annurev.micro.112408.134040. Annu Rev Microbiol. 2011. PMID: 21801026 Review.
-
The biology of enhancer-dependent transcriptional regulation in bacteria: insights from genome sequences.FEMS Microbiol Lett. 2000 May 1;186(1):1-9. doi: 10.1111/j.1574-6968.2000.tb09074.x. FEMS Microbiol Lett. 2000. PMID: 10779705 Review.
Cited by
-
Extensive diversity in RNA termination and regulation revealed by transcriptome mapping for the Lyme pathogen Borrelia burgdorferi.Nat Commun. 2023 Jul 4;14(1):3931. doi: 10.1038/s41467-023-39576-1. Nat Commun. 2023. PMID: 37402717 Free PMC article.
-
Gene Regulation and Transcriptomics.Curr Issues Mol Biol. 2021;42:223-266. doi: 10.21775/cimb.042.223. Epub 2020 Dec 10. Curr Issues Mol Biol. 2021. PMID: 33300497 Free PMC article.
-
DksA-dependent regulation of RpoS contributes to Borrelia burgdorferi tick-borne transmission and mammalian infectivity.PLoS Pathog. 2021 Feb 18;17(2):e1009072. doi: 10.1371/journal.ppat.1009072. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33600418 Free PMC article.
-
c-di-GMP regulates activity of the PlzA RNA chaperone from the Lyme disease spirochete.Mol Microbiol. 2023 Jun;119(6):711-727. doi: 10.1111/mmi.15066. Epub 2023 Apr 21. Mol Microbiol. 2023. PMID: 37086029 Free PMC article.
-
bb0689 contributes to the virulence of Borrelia burgdorferi in a murine model of Lyme disease.Infect Immun. 2025 Jan 31;93(1):e0045924. doi: 10.1128/iai.00459-24. Epub 2024 Dec 16. Infect Immun. 2025. PMID: 39679711 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

