Influenza A(H1N1)pdm09 infection and viral load analysis in patients with different clinical presentations
- PMID: 32428082
- PMCID: PMC7233266
- DOI: 10.1590/0074-02760200009
Influenza A(H1N1)pdm09 infection and viral load analysis in patients with different clinical presentations
Abstract
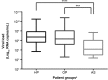
BACKGROUND Influenza viral load (VL) can be a decisive factor in determining the antiviral efficacy in viral clearance. OBJECTIVE This study aimed to evaluate the rate of infection and the role of influenza VL on the clinical spectrum of illnesses among different patient groups attended at a tertiary hospital in Brazil. METHODS Samples were collected from patients presenting acute respiratory infection from 2009 to 2013. Overall, 2262 samples were analysed and distributed into three groups: (i) asymptomatic (AS); (ii) symptomatic outpatients (OP); and (iii) hospitalised patients (HP). VL (expressed in Log10 RNA copies/mL) was calculated through a quantitative real-time one-step reverse transcription-polymerase chain reaction (RT-PCR) assay aimed at the M gene, with human RNAseP target as internal control and normalising gene of threshold cycle values. FINDINGS A total of 162 (7.16%) H1N1pdm09 positive samples were analysed. Patients aged from 0.08 to 77 years old [median ± standard deviation (SD): 12.5 ± 20.54]. Children with 5 to 11 years old presented the highest detection (p < 0.0001). AS patients had the lowest VL, with a significant difference when compared with symptomatic patients (p = 0.0003). A higher VL was observed within two days of disease onset. Ten patients (HP group) received antiviral treatment and were followed up and presented a mean initial VL of 6.64 ± 1.82. A complete viral clearance for 50% of these patients was reached after 12 days of treatment. MAIN CONCLUSIONS It is important to evaluate AS patients as potential spreaders, as viral shedding was still present, even at lower VL. Our results suggest that patients with underlying diseases and severe clinical symptoms may be considered for prolonged viral treatment.
Figures

References
-
- World Health Organization Recommended composition of influenza virus vaccines for use in the 2020 Southern Hemisphere influenza season. 2019. https://www.who.int/influenza/vaccines/virus/recommendations/2020_south/...
-
- World Health Organization Influenza virus infections in humans (February 2014) homepage on the Internet. 2014. https://www.who.int/influenza/human_animal_interface/virology_laboratori...
-
- Ministério da Saúde Informe técnico: 20ª Campanha Nacional de Vacinação contra a Influenza. homepage on the Internet. 2018. http://portalarquivos2.saude.gov.br/images/pdf/2018/abril/18/Informe-Cp-...
-
- Centers for Disease Control and Prevention Flu symptoms & complications. homepage on the Internet. 2019. https://www.cdc.gov/flu/symptoms/symptoms.htm
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

