Treatment Potential for LCA5-Associated Leber Congenital Amaurosis
- PMID: 32428231
- PMCID: PMC7405811
- DOI: 10.1167/iovs.61.5.30
Treatment Potential for LCA5-Associated Leber Congenital Amaurosis
Abstract
Purpose: To determine the therapeutic window for gene augmentation for Leber congenital amaurosis (LCA) associated with mutations in LCA5.
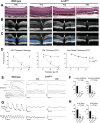
Methods: Five patients (ages 6-31) with LCA and biallelic LCA5 mutations underwent an ophthalmic examination including optical coherence tomography (SD-OCT), full-field stimulus testing (FST), and pupillometry. The time course of photoreceptor degeneration in the Lca5gt/gt mouse model and the efficacy of subretinal gene augmentation therapy with AAV8-hLCA5 delivered at postnatal day 5 (P5) (early, n = 11 eyes), P15 (mid, n = 14), and P30 (late, n = 13) were assessed using SD-OCT, histologic study, electroretinography (ERG), and pupillometry. Comparisons were made with the human disease.
Results: Patients with LCA5-LCA showed a maculopathy with detectable outer nuclear layer (ONL) in the pericentral retina and at least 4 log units of dark-adapted sensitivity loss. The Lca5gt/gt mouse has a similarly severe and rapid photoreceptor degeneration. The ONL became progressively thinner and was undetectable by P60. Rod- and cone-mediated ERGs were severely reduced in amplitudes at P30 and became nondetectable by P60. Subretinal AAV8-hLCA5 administered to Lca5gt/gt mice at P5 and P15, but not at P30, resulted in structural and functional rescue.
Conclusions: LCA5-LCA is a particularly severe form of LCA that was recapitulated in the Lca5gt/gt mouse. Gene augmentation resulted in structural and functional rescue in the Lca5gt/gt mouse if delivered before P30. Retained photoreceptors were visible within the central retina in all patients with LCA5-LCA, at a level equivalent to that observed in rescued Lca5gt/gt mice, suggesting a window of opportunity for the treatment of patients with LCA5-LCA.
Conflict of interest statement
Disclosure:
Figures





References
-
- Dharmaraj S, Li Y, Robitaille J, et al. .. A novel locus for Leber congenital amaurosis maps to chromosome 6q. Am J Hum Genet. 2000; 66: 319–326. - PubMed
-
- den Hollander AI, Koenekoop RK, Mohamed MD, et al. .. Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat Genet. 2007; 39: 889–895. - PubMed
-
- Gerber S, Hanein S, Perrault I, et al. .. Mutations in LCA5 are an uncommon cause of Leber congenital amaurosis (LCA) type II. Hum Mutat. 2007; 28: 1245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

