Prebiotic Nucleoside Synthesis: The Selectivity of Simplicity
- PMID: 32428355
- PMCID: PMC7756251
- DOI: 10.1002/chem.202001513
Prebiotic Nucleoside Synthesis: The Selectivity of Simplicity
Abstract
Ever since the discovery of nucleic acids 150 years ago,[1] major achievements have been made in understanding and decrypting the fascinating scientific questions of the genetic code.[2] However, the most fundamental question about the origin and the evolution of the genetic code remains a mystery. How did nature manage to build up such intriguingly complex molecules able to encode structure and function from simple building blocks? What conditions were required? How could the precursors survive the unhostile environment of early Earth? Over the past decades, promising synthetic concepts were proposed providing clarity in the field of prebiotic nucleic acid research. In this Minireview, we show the current status and various approaches to answer these fascinating questions.
Keywords: deoxyribonucleoside synthesis; heterocycles; nucleoside synthesis; origins of life; prebiotic chemistry.
© 2020 The Authors. Published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

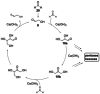

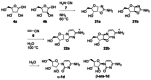


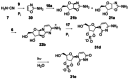

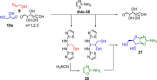
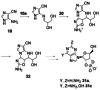
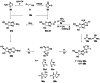
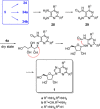
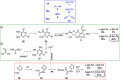

References
-
- Miescher F., Med. Chem. Unters. 1871, 4, 441–460.
-
- None
-
- A. R. Todd, Nobel Lecture, 1957;
-
- A. Kronberg, Nobel Lecture, 1959;
-
- R. W. Holley, Nobel Lecture, 1968;
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

