The Human Cytochrome c Domain-Swapped Dimer Facilitates Tight Regulation of Intrinsic Apoptosis
- PMID: 32428404
- PMCID: PMC7291863
- DOI: 10.1021/acs.biochem.0c00326
The Human Cytochrome c Domain-Swapped Dimer Facilitates Tight Regulation of Intrinsic Apoptosis
Abstract
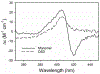
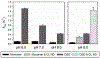
Oxidation of cardiolipin (CL) by cytochrome c (cytc) has been proposed to initiate the intrinsic pathway of apoptosis. Domain-swapped dimer (DSD) conformations of cytc have been reported both by our laboratory and by others. The DSD is an alternate conformer of cytc that could oxygenate CL early in apoptosis. We demonstrate here that the cytc DSD has a set of properties that would provide tighter regulation of the intrinsic pathway. We show that the human DSD is kinetically more stable than horse and yeast DSDs. Circular dichroism data indicate that the DSD has a less asymmetric heme environment, similar to that seen when the monomeric protein binds to CL vesicles at high lipid:protein ratios. The dimer undergoes the alkaline conformational transition near pH 7.0, 2.5 pH units lower than that of the monomer. Data from fluorescence correlation spectroscopy and fluorescence anisotropy suggest that the alkaline transition of the DSD may act as a switch from a high affinity for CL nanodiscs at pH 7.4 to a much lower affinity at pH 8.0. Additionally, the peroxidase activity of the human DSD increases 7-fold compared to that of the monomer at pH 7 and 8, but by 14-fold at pH 6 when mixed Met80/H2O ligation replaces the lysine ligation of the alkaline state. We also present data that indicate that cytc binding shows a cooperative effect as the concentration of cytc is increased. The DSD appears to have evolved into a pH-inducible switch that provides a means to control activation of apoptosis near pH 7.0.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


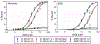

Similar articles
-
Alkaline State of the Domain-Swapped Dimer of Human Cytochrome c: A Conformational Switch for Apoptotic Peroxidase Activity.J Am Chem Soc. 2022 Nov 23;144(46):21184-21195. doi: 10.1021/jacs.2c08325. Epub 2022 Nov 8. J Am Chem Soc. 2022. PMID: 36346995 Free PMC article.
-
Reductive nitrosylation of the cardiolipin-ferric cytochrome c complex.IUBMB Life. 2014 Jun;66(6):438-47. doi: 10.1002/iub.1283. Epub 2014 Jun 30. IUBMB Life. 2014. PMID: 24979722
-
Cardiolipin modulates allosterically the nitrite reductase activity of horse heart cytochrome c.J Biol Inorg Chem. 2014 Oct;19(7):1195-201. doi: 10.1007/s00775-014-1175-9. Epub 2014 Jun 27. J Biol Inorg Chem. 2014. PMID: 24969400
-
Cardiolipin-cytochrome c complex: Switching cytochrome c from an electron-transfer shuttle to a myoglobin- and a peroxidase-like heme-protein.IUBMB Life. 2015 Feb;67(2):98-109. doi: 10.1002/iub.1350. Epub 2015 Apr 9. IUBMB Life. 2015. PMID: 25857294 Review.
-
Structural transformations of cytochrome c upon interaction with cardiolipin.Chem Phys Lipids. 2014 Apr;179:57-63. doi: 10.1016/j.chemphyslip.2013.11.002. Epub 2013 Nov 16. Chem Phys Lipids. 2014. PMID: 24252639 Free PMC article. Review.
Cited by
-
Calcium-induced release of cytochrome c from cardiolipin nanodisks: Implications for apoptosis.Biochim Biophys Acta Biomembr. 2021 Dec 1;1863(12):183722. doi: 10.1016/j.bbamem.2021.183722. Epub 2021 Aug 14. Biochim Biophys Acta Biomembr. 2021. PMID: 34400138 Free PMC article.
-
Alkaline State of the Domain-Swapped Dimer of Human Cytochrome c: A Conformational Switch for Apoptotic Peroxidase Activity.J Am Chem Soc. 2022 Nov 23;144(46):21184-21195. doi: 10.1021/jacs.2c08325. Epub 2022 Nov 8. J Am Chem Soc. 2022. PMID: 36346995 Free PMC article.
-
Order-to-Disorder and Disorder-to-Order Transitions of Proteins upon Binding to Phospholipid Membranes: Common Ground and Dissimilarities.Biomolecules. 2025 Jan 30;15(2):198. doi: 10.3390/biom15020198. Biomolecules. 2025. PMID: 40001501 Free PMC article. Review.
-
Integrative biophysical characterization of molecular interactions: A case study with the sfGFP-nanobody complex.Anal Biochem. 2025 Aug;703:115859. doi: 10.1016/j.ab.2025.115859. Epub 2025 Apr 7. Anal Biochem. 2025. PMID: 40204056 Free PMC article.
-
Effect on intrinsic peroxidase activity of substituting coevolved residues from Ω-loop C of human cytochrome c into yeast iso-1-cytochrome c.J Inorg Biochem. 2022 Jul;232:111819. doi: 10.1016/j.jinorgbio.2022.111819. Epub 2022 Apr 6. J Inorg Biochem. 2022. PMID: 35428021 Free PMC article.
References
-
- Dickerson RE, and Timkovich R (1975) Cytochromes c, In The Enzymes (Boyer PD, Ed.) 3rd ed., pp 397–547, Academic Press, New York.
-
- Moore GR, and Pettigrew GW (1990) Cytochromes c: Evolutionary, Structural and Physicochemical Aspects, Springer-Verlag, New York; 10.1007/978-3-642-74536-2. - DOI
-
- Deacon OM, Karsisiotis AI, Moreno-Chicano T, Hough MA, Macdonald C, Blumenschein TMA, Wilson MT, Moore GR, and Worrall JAR (2017) Heightened dynamics of the oxidized Y48H variant of human cytochrome c increases its peroxidatic activity, Biochemistry 56, 6111–6124. 10.1021/acs.biochem.7b00890. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

