The Human Cytochrome c Domain-Swapped Dimer Facilitates Tight Regulation of Intrinsic Apoptosis
- PMID: 32428404
- PMCID: PMC7291863
- DOI: 10.1021/acs.biochem.0c00326
The Human Cytochrome c Domain-Swapped Dimer Facilitates Tight Regulation of Intrinsic Apoptosis
Abstract
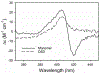
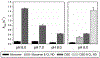
Oxidation of cardiolipin (CL) by cytochrome c (cytc) has been proposed to initiate the intrinsic pathway of apoptosis. Domain-swapped dimer (DSD) conformations of cytc have been reported both by our laboratory and by others. The DSD is an alternate conformer of cytc that could oxygenate CL early in apoptosis. We demonstrate here that the cytc DSD has a set of properties that would provide tighter regulation of the intrinsic pathway. We show that the human DSD is kinetically more stable than horse and yeast DSDs. Circular dichroism data indicate that the DSD has a less asymmetric heme environment, similar to that seen when the monomeric protein binds to CL vesicles at high lipid:protein ratios. The dimer undergoes the alkaline conformational transition near pH 7.0, 2.5 pH units lower than that of the monomer. Data from fluorescence correlation spectroscopy and fluorescence anisotropy suggest that the alkaline transition of the DSD may act as a switch from a high affinity for CL nanodiscs at pH 7.4 to a much lower affinity at pH 8.0. Additionally, the peroxidase activity of the human DSD increases 7-fold compared to that of the monomer at pH 7 and 8, but by 14-fold at pH 6 when mixed Met80/H2O ligation replaces the lysine ligation of the alkaline state. We also present data that indicate that cytc binding shows a cooperative effect as the concentration of cytc is increased. The DSD appears to have evolved into a pH-inducible switch that provides a means to control activation of apoptosis near pH 7.0.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


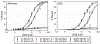

References
-
- Dickerson RE, and Timkovich R (1975) Cytochromes c, In The Enzymes (Boyer PD, Ed.) 3rd ed., pp 397–547, Academic Press, New York.
-
- Moore GR, and Pettigrew GW (1990) Cytochromes c: Evolutionary, Structural and Physicochemical Aspects, Springer-Verlag, New York; 10.1007/978-3-642-74536-2. - DOI
-
- Deacon OM, Karsisiotis AI, Moreno-Chicano T, Hough MA, Macdonald C, Blumenschein TMA, Wilson MT, Moore GR, and Worrall JAR (2017) Heightened dynamics of the oxidized Y48H variant of human cytochrome c increases its peroxidatic activity, Biochemistry 56, 6111–6124. 10.1021/acs.biochem.7b00890. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

