Tracing the Evolutionary Origin of Desmosomes
- PMID: 32428495
- PMCID: PMC7310670
- DOI: 10.1016/j.cub.2020.03.047
Tracing the Evolutionary Origin of Desmosomes
Abstract
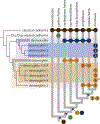
Cadherin-based cell-cell junctions help metazoans form polarized sheets of cells, which are necessary for the development of organs and the compartmentalization of functions. The components of the protein complexes that generate cadherin-based junctions have ancient origins, with conserved elements shared between animals as diverse as sponges and vertebrates. In invertebrates, the formation and function of epithelial sheets depends on classical cadherin-containing adherens junctions, which link actin to the plasma membrane through α-, β- and p120 catenins. Vertebrates also have a new type of cadherin-based intercellular junction called the desmosome, which allowed for the creation of more complex and effective tissue barriers against environmental stress. While desmosomes have a molecular blueprint that is similar to that of adherens junctions, desmosomal cadherins - called desmogleins and desmocollins - link intermediate filaments (IFs) rather than actin to the plasma membrane through protein complexes comprising relatives of β-catenin (plakoglobin) and p120 catenin (plakophilins). In turn, desmosomal catenins interact with members of the IF-binding plakin family to create the desmosome-IF linking complex. In this Minireview, we discuss when and how desmosomal components evolved, and how their ability to anchor the highly elastic and tough IF cytoskeleton endowed vertebrates with robust tissues capable of not only resisting but also properly responding to environmental stress.
Copyright © 2020 Elsevier Inc. All rights reserved.
Figures



References
-
- Brunet T, Larson BT, Linden TA, Vermeij MJA, McDonald K, and King N (2019). Light-regulated collective contractility in a multicellular choanoflagellate. Science 366, 326–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

