Pharmacokinetics of Gepotidacin in Renal Impairment
- PMID: 32429000
- PMCID: PMC7384084
- DOI: 10.1002/cpdd.807
Pharmacokinetics of Gepotidacin in Renal Impairment
Abstract
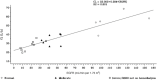
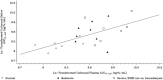
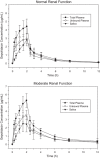
Gepotidacin is a novel triazaacenaphthylene bacterial topoisomerase inhibitor. In this phase 1, nonrandomized, open-label, parallel-group, multicenter, multipart study, the pharmacokinetics, safety, and tolerability of a single intravenous (IV) dose of gepotidacin 750 mg over 2 hours were evaluated in subjects with normal renal function, in those with moderate and severe renal impairment, and in end-stage renal disease (ESRD) on and not on dialysis. Administration of IV gepotidacin 750 mg was safe and generally tolerated in the study subjects. Dosing in severe renal impairment with and without hemodialysis resulted in significant increases in plasma drug levels and decreases in clearance. The geometric mean elimination half-life (t½ ) was minimally impacted (range 9.45 to 11.5 hours) in all the renal-impairment groups relative to normal renal function. Regardless of renal function, urine gepotidacin concentrations remained considerably high over a 12-hour period. Saliva concentrations displayed a linear relationship with plasma concentrations. The t½ in saliva was not impacted in the moderate-impairment and ESRD subjects and was comparable to t½ in plasma. Over a 4-hour dialysis, approximately 6% of the gepotidacin dose was removed. Overall, subjects with severe renal impairment and ESRD with and without hemodialysis may require adjustment in dose or dosing frequency.
Trial registration: ClinicalTrials.gov NCT02729038.
Keywords: end-stage renal disease; gepotidacin; pharmacokinetics; physiologically based pharmacokinetic modeling; renal impairment; safety.
© 2020 GlaxoSmithKline. Clinical Pharmacology in Drug Development published by Wiley Periodicals LLC on behalf of American College of Clinical Pharmacology.
Conflict of interest statement
M.H., C.T., A.R., D.N., G.T., and E.D. were employees of GSK and hold company stock. H.A., R.P., and T.M. were the study investigators funded by GSK during the conduct of the study. All authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Figures

References
-
- Bax BD, Chan PF, Eggleston DS, et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature. 2010;466(7309):935‐940. - PubMed
-
- Negash K, Andonian C, Felgate C, et al. The metabolism and disposition of GSK2140944 in healthy human subjects. Xenobiotica. 2016;46(8):683‐702. - PubMed
-
- Hossain M, Zhou M, Tiffany C, Dumont E, Darpo B. A phase I, randomized, double‐blinded, placebo‐ and moxifloxacin‐controlled, four‐period crossover study to evaluate the effect of gepotidacin on cardiac conduction as assessed by 12‐lead electrocardiogram in healthy volunteers. Antimicrob Agents Chemother. 61:e02385‐16. 10.1128/AAC.02385-16/bib> - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

