Biosignatures Associated with Freshwater Microbialites
- PMID: 32429118
- PMCID: PMC7281397
- DOI: 10.3390/life10050066
Biosignatures Associated with Freshwater Microbialites
Abstract
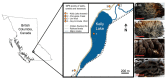
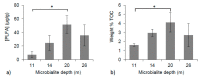
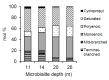
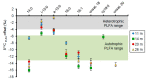
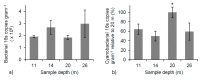
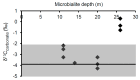
Freshwater microbialites (i.e., lithifying microbial mats) are quite rare in northern latitudes of the North American continent, with two lakes (Pavilion and Kelly Lakes) of southeastern BC containing a morphological variety of such structures. We investigated Kelly Lake microbialites using carbon isotope systematics, phospholipid fatty acids (PLFAs) and quantitative PCR to obtain biosignatures associated with microbial metabolism. δ13CDIC values (mean δ13CDIC -4.9 ± 1.1‱, n = 8) were not in isotopic equilibrium with the atmosphere; however, they do indicate 13C-depleted inorganic carbon into Kelly Lake. The values of carbonates on microbialite surfaces (δ13C) fell within the range predicted for equilibrium precipitation from ambient lake water δ13CDIC (-2.2 to -5.3‱). Deep microbialites (26 m) had an enriched δ13Ccarb value of -0.3 ± 0.5‱, which is a signature of photoautotrophy. The deeper microbialites (>20 m) had higher biomass estimates (via PLFAs), and a greater relative abundance of cyanobacteria (measured by 16S copies via qPCR). The majority of PLFAs constituted monounsaturated and saturated PLFAs, which is consistent with gram-negative bacteria, including cyanobacteria. The central PLFA δ13C values were highly depleted (-9.3 to -15.7‱) relative to δ13C values of bulk organic matter, suggesting a predominance of photoautotrophy. A heterotrophic signature was also detected via the depleted iso- and anteiso-15:0 lipids (-3.2 to -5.2‱). Based on our carbonate isotopic biosignatures, PLFA, and qPCR measurements, photoautotrophy is enriched in the microbialites of Kelly Lake. This photoautotrophy enrichment is consistent with the microbialites of neighboring Pavilion Lake. This indication of photoautotrophy within Kelly Lake at its deepest depths raises new insights into the limits of measurable carbonate isotopic biosignatures under light and nutrient limitations.
Keywords: Kelly Lake; Pavilion Lake; biosignatures; microbialites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Andres M.S., Sumner D.Y., Reid R.P., Swart P.K. Isotopic fingerprints of microbial respiration in aragonite from Bahamian stromatolites. Geology. 2006;34:973–976. doi: 10.1130/G22859A.1. - DOI
-
- Breitbart M., Hoare A., Nitti A., Siefert J., Haynes M., Dinsdale E., Edwards R., Souza V., Rohwer F., Hollander D. Metagenomic and stable isotopic analyses of modern freshwater microbialites in Cuatro Ciénegas, Mexico. Environ. Microbiol. 2009;11:16–34. doi: 10.1111/j.1462-2920.2008.01725.x. - DOI - PubMed
-
- Kempe S., Kazmierczak J., Landmann G., Konuk T., Reimer A., Lipp A. Largest known microbialites discovered in Lake Van, Turkey. Nature. 1991;349:605–608. doi: 10.1038/349605a0. - DOI
LinkOut - more resources
Full Text Sources

