M2 Macrophages Infiltrating Epithelial Ovarian Cancer Express MDR1: A Feature That May Account for the Poor Prognosis
- PMID: 32429133
- PMCID: PMC7290705
- DOI: 10.3390/cells9051224
M2 Macrophages Infiltrating Epithelial Ovarian Cancer Express MDR1: A Feature That May Account for the Poor Prognosis
Abstract
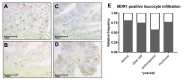
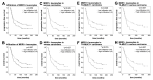
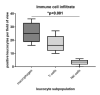
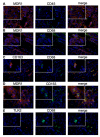
Multi drug resistance protein 1 (MDR1) expression on tumor cells has been widely investigated in context of drug resistance. However, the role of MDR1 on the immune cell infiltrate of solid tumors remains unknown. The aim of this study was to analyze the prognostic significance of a MDR1+ immune cell infiltrate in epithelial ovarian cancer (EOC) and to identify the MDR1+ leucocyte subpopulation. MDR1 expression was analyzed by immunohistochemistry in 156 EOC samples. In addition to MDR1+ cancer cells, we detected a MDR1+ leucocyte infiltrate (high infiltrate >4 leucocytes per field of view). Correlations and survival analyses were calculated. To identify immune cell subpopulations immunofluorescence double staining was performed. The MDR1+ leucocyte infiltrate was associated with human epidermal growth factor receptor 2 (HER2) (cc = 0.258, p = 0.005) and tumor-associated mucin 1 (TA-MUC1) (cc = 0.202, p = 0.022) expression on cancer cells. A high MDR1+ leucocyte infiltrate was associated with impaired survival, especially in patients whose carcinoma showed either serous histology (median OS 28.80 vs. 50.64 months, p = 0.027, n = 91) or TA-MUC1 expression (median OS 30.60 vs. 63.36 months, p = 0.015, n = 110). Similar findings for PFS suggest an influence of MDR1+ immune cells on the development of chemoresistance. A Cox regression analysis confirmed the independency of a high MDR1+ leucocyte infiltrate as prognostic factor. M2 macrophages were identified as main part of the MDR1+ leucocyte infiltrate expressing MDR1 as well as the M2 marker CD163 and the pan-macrophage marker CD68. Infiltration of MDR1+ leucocytes, mostly M2 macrophages, is associated with poor prognosis of EOC patients. Further understanding of the interaction of M2 macrophages, MDR1 and TA-MUC1 appears to be a key aspect to overcome chemoresistance in ovarian cancer.
Keywords: M2 Macrophages; MDR1; TA-MUC1; ovarian cancer; prognosis.
Conflict of interest statement
T.K. holds stock of Roche AG and his relative is employed at Roche AG. A.H. has received a research grant from the “Walter Schulz” foundation and advisory board, speech honoraria and travel expenses from Roche and Pfizer. A.B. has received advisory board and honoraria from AstraZeneca, Clovis, Roche and Tesaro. Research support, advisory board, honoraria, and travel expenses from AstraZeneca, Clovis, Medac, MSD, Novartis, PharmaMar, Roche, Sensor Kinesis, Tesaro, Teva have been received by S.M. and from AstraZeneca, Medac, PharmaMar, Roche, Tesaro by F.T. All other authors declare no conflict of interest.
Figures

References
-
- Ciucci A., Zannoni G.F., Buttarelli M., Martinelli E., Mascilini F., Petrillo M., Ferrandina G., Scambia G., Gallo D. Ovarian low and high grade serous carcinomas: Hidden divergent features in the tumor microenvironment. Oncotarget. 2016;7:68033–68043. doi: 10.18632/oncotarget.10797. - DOI - PMC - PubMed
-
- Colombo N., Sessa C., Bois A.D., Ledermann J., McCluggage W.G., McNeish I., Morice P., Pignata S., Ray-Coquard I., Vergote I., et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int. J. Gynecol. Cancer. 2019 doi: 10.1136/ijgc-2019-000308. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

