Direct Enantiomeric Separation and Determination of Hexythiazox Enantiomers in Environment and Vegetable by Reverse-Phase High-Performance Liquid Chromatography
- PMID: 32429166
- PMCID: PMC7277754
- DOI: 10.3390/ijerph17103453
Direct Enantiomeric Separation and Determination of Hexythiazox Enantiomers in Environment and Vegetable by Reverse-Phase High-Performance Liquid Chromatography
Abstract
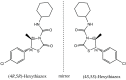
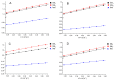
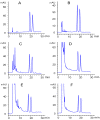
In the present study, the direct enantiomeric separation of hexythiazox enantiomers on Lux cellulose-1, Lux cellulose-2, Lux cellulose-3, Lux cellulose-4, Lux amylose-1 and Chirapak IC chiral columns were carefully investigated by reverse-phase high-performance liquid chromatography (RP-HPLC). Acetonitrile/water and methanol/water were used as mobile phase at a flow rate of 0.8 mL·min-1. The effects of chiral stationary phase, temperature, thermodynamic parameters, mobile phase component and mobile phase ratio on hexythiazox enantiomers separation were fully evaluated. Hexythiazox enantiomers received a baseline separation on the Lux cellulose-3 column with a maximum resolution of Rs = 2.09 (methanol/water) and Rs = 2.74 (acetonitrile/water), respectively. Partial separations were achieved on other five chiral columns. Furthermore, Lux amylose-1 and Chirapak IC had no separation ability for hexythiazox enantiomers when methanol/water was used as mobile phase. Temperature study indicated that the capacity factor (k) and resolution factor (Rs) decreased with column temperature increasing from 10 °C to 40 °C. The enthalpy (ΔH) and entropy (ΔS) involved in hexythiazox separation were also calculated and demonstrated the lower temperature contributed to better separation resolution. Moreover, the residue analytical method for hexythiazox enantiomers in the environment (soil and water) and vegetable (cucumber, cabbage and tomato) were also established with reliable accuracy and precision under reverse-phase HPLC condition. Such results provided a baseline separation method for hexythiazox enantiomers under reverse-phase conditions and contributed to an environmental and health risk assessment of hexythiazox at enantiomer level.
Keywords: enantiomeric separation; environment; hexythiazox; residue analysis; vegetable.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the writing of the manuscript and in the decision to publish the results.
Figures

Similar articles
-
Chiral Separation and Determination of Etoxazole Enantiomers in Vegetables by Normal-Phase and Reverse-Phase High Performance Liquid Chromatography.Molecules. 2020 Jul 9;25(14):3134. doi: 10.3390/molecules25143134. Molecules. 2020. PMID: 32659902 Free PMC article.
-
Enantiomeric Separation and Degradation of Benoxacor Enantiomers in Horticultural Soil by Normal-Phase and Reversed-Phase High Performance Liquid Chromatography.Int J Mol Sci. 2023 May 17;24(10):8887. doi: 10.3390/ijms24108887. Int J Mol Sci. 2023. PMID: 37240233 Free PMC article.
-
Enantiomeric separation of type I and type II pyrethroid insecticides with different chiral stationary phases by reversed-phase high-performance liquid chromatography.Chirality. 2018 Apr;30(4):420-431. doi: 10.1002/chir.22801. Epub 2017 Dec 23. Chirality. 2018. PMID: 29274232
-
Simultaneous determination of enantiomeric and organic impurities of vildagliptin on a cellulose tris(3-chloro-4-methylphenylcarbamate) column under revered-phase conditions.J Pharm Biomed Anal. 2023 Sep 20;234:115495. doi: 10.1016/j.jpba.2023.115495. Epub 2023 Jun 9. J Pharm Biomed Anal. 2023. PMID: 37343452
-
Simultaneous Determination of Escitalopram Impurities including the R-enantiomer on a Cellulose tris(3,5-Dimethylphenylcarbamate)-Based Chiral Column in Reversed-Phase Mode.Molecules. 2022 Dec 17;27(24):9022. doi: 10.3390/molecules27249022. Molecules. 2022. PMID: 36558157 Free PMC article.
Cited by
-
[Determination of myclobutanil enantiomers in wheat and its processed products by ultraperformance liquid chromatography-tandem mass spectrometry based on a chiral stationary phase].Se Pu. 2021 Jul 8;39(7):702-707. doi: 10.3724/SP.J.1123.2021.03001. Se Pu. 2021. PMID: 34227367 Free PMC article. Chinese.
References
-
- Thwaite W.G. Resistance to Clofentezine and Hexythiazox in Panonychus-Ulmi from Apples in Australia. Exp. Appl. Acarol. 1991;11:73–80. doi: 10.1007/BF01193730. - DOI
-
- Ganjisaffar F., Perring T.M. Effects of the miticide hexythiazox on biology of Galendromus flumenis (Acari: Phytoseiidae) Int. J. Acarol. 2016;43:169–172. doi: 10.1080/01647954.2016.1256348. - DOI
-
- Abdourahime H., Anastassiadou M., Brancato A., Brocca D., Carrasco Cabrera L., De Lentdecker C., Ferreira L., Greco L., Jarrah S., Kardassi D., et al. Review of the existing maximum residue levels for hexythiazox according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2019;17:e05559. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

