Peritoneal Fluid Cytokines Reveal New Insights of Endometriosis Subphenotypes
- PMID: 32429215
- PMCID: PMC7278942
- DOI: 10.3390/ijms21103515
Peritoneal Fluid Cytokines Reveal New Insights of Endometriosis Subphenotypes
Abstract
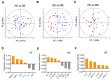
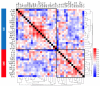
Endometriosis is a common inflammatory gynecological disorder which causes pelvic scarring, pain, and infertility, characterized by the implantation of endometrial-like lesions outside the uterus. The peritoneum, ovaries, and deep soft tissues are the commonly involved sites, and endometriotic lesions can be classified into three subphenotypes: superficial peritoneal endometriosis (PE), ovarian endometrioma (OE), and deep infiltrating endometriosis (DIE). In 132 women diagnosed laparoscopically with and without endometriosis (n = 73, 59 respectively), and stratified into PE, OE, and DIE, peritoneal fluids (PF) were characterized for 48 cytokines by using multiplex immunoassays. Partial-least-squares-regression analysis revealed distinct subphenotype cytokine signatures-a six-cytokine signature distinguishing PE from OE, a seven-cytokine signature distinguishing OE from DIE, and a six-cytokine-signature distinguishing PE from DIE-each associated with different patterns of biological processes, signaling events, and immunology. These signatures describe endometriosis better than disease stages (p < 0.0001). Pathway analysis revealed the association of ERK1 and 2, AKT, MAPK, and STAT4 linked to angiogenesis, cell proliferation, migration, and inflammation in the subphenotypes. These data shed new insights on the pathophysiology of endometriosis subphenotypes, with the potential to exploit the cytokine signatures to stratify endometriosis patients for targeted therapies and biomarker discovery.
Keywords: cytokines; endometriosis; microenvironment; peritoneal fluid; precision medicine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

