Functional Block of Interleukin-6 Reduces a Bone Pain Marker but Not Bone Loss in Hindlimb-Unloaded Mice
- PMID: 32429268
- PMCID: PMC7278999
- DOI: 10.3390/ijms21103521
Functional Block of Interleukin-6 Reduces a Bone Pain Marker but Not Bone Loss in Hindlimb-Unloaded Mice
Abstract
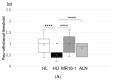
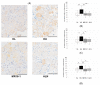
Interleukin-6 (IL-6) is widely accepted to stimulate osteoclasts. Our aim in this study was to examine whether the inhibitory effect of IL-6 on bone loss and skeletal pain associated with osteoporosis in hindlimb-unloaded (HU) mice in comparison with bisphosphonate. Eight-week-old male ddY mice were tail suspended for 2 weeks. Starting immediately after reload, vehicle (HU group), alendronate (HU-ALN group), or anti-IL-6 receptor antibody (HU-IL-6i group) was injected subcutaneously. After a 2-week treatment, pain-related behavior was examined using von Frey filaments. The bilateral distal femoral and proximal tibial metaphyses were analyzed three-dimensionally with micro-computed tomography. Calcitonin gene-related peptide (CGRP) expressions in dorsal root ganglion (DRG) neurons innervating the hindlimbs were examined using immunohistochemistry. HU mice with tail suspension developed bone loss. The HU mice showed mechanical hyperalgesia in the hindlimbs and increased CGRP immunoreactive neurons in the L3-5 DRG. Treatment with IL-6i and ALN prevented HU-induced mechanical hyperalgesia and upregulation of CGRP expressions in DRG neurons. Furthermore, ALN but not IL-6i prevented HU-induced bone loss. In summary, treatment with IL-6i prevented mechanical hyperalgesia in hindlimbs and suppressed CGRP expressions in DRG neurons of osteoporotic models. The novelty of this research suggests that IL-6 is one of the causes of immobility-induced osteoporotic pain regardless improvement of bone loss.
Keywords: CGRP; hindlimb-unloading; interleukin-6; osteoporosis; osteoporotic pain; tail suspension.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Interleukin-6 Inhibitor Suppresses Hyperalgesia Without Improvement in Osteoporosis in a Mouse Pain Model of Osteoporosis.Calcif Tissue Int. 2019 Jun;104(6):658-666. doi: 10.1007/s00223-019-00521-4. Epub 2019 Jan 21. Calcif Tissue Int. 2019. PMID: 30666355
-
Alendronate inhibits hyperalgesia and suppresses neuropeptide markers of pain in a mouse model of osteoporosis.J Orthop Sci. 2017 Jul;22(4):771-777. doi: 10.1016/j.jos.2017.02.001. Epub 2017 Feb 28. J Orthop Sci. 2017. PMID: 28258808
-
Prevention of bone loss and improvement of pain-related behavior in hind limb-unloaded mice by administration of teriparatide and bisphosphonate.Mod Rheumatol. 2021 May;31(3):733-742. doi: 10.1080/14397595.2020.1782592. Epub 2020 Jul 10. Mod Rheumatol. 2021. PMID: 32646253
-
The effects of bisphosphonate on pain-related behavior and immunohistochemical analyses in hindlimb-unloaded mice.J Orthop Sci. 2018 Nov;23(6):1063-1069. doi: 10.1016/j.jos.2018.06.002. Epub 2018 Jul 4. J Orthop Sci. 2018. PMID: 30431005
-
Teriparatide improves pain-related behavior and prevents bone loss in ovariectomized mice.J Orthop Surg (Hong Kong). 2020 Jan-Apr;28(1):2309499019893194. doi: 10.1177/2309499019893194. J Orthop Surg (Hong Kong). 2020. PMID: 31833446
Cited by
-
CTLA-4Ig Improves Hyperalgesia in a Mouse Model of Osteoporosis.Int J Mol Sci. 2020 Dec 13;21(24):9479. doi: 10.3390/ijms21249479. Int J Mol Sci. 2020. PMID: 33322156 Free PMC article.
-
Effects of Biological/Targeted Therapies on Bone Mineral Density in Inflammatory Arthritis.Int J Mol Sci. 2022 Apr 8;23(8):4111. doi: 10.3390/ijms23084111. Int J Mol Sci. 2022. PMID: 35456929 Free PMC article. Review.
-
Chronic Pain in Musculoskeletal Diseases: Do You Know Your Enemy?J Clin Med. 2022 May 6;11(9):2609. doi: 10.3390/jcm11092609. J Clin Med. 2022. PMID: 35566735 Free PMC article. Review.
-
Osteosarcopenia and Pain: Do We Have a Way Out?Biomedicines. 2023 Apr 26;11(5):1285. doi: 10.3390/biomedicines11051285. Biomedicines. 2023. PMID: 37238956 Free PMC article. Review.
-
Microgravity-Related Changes in Bone Density and Treatment Options: A Systematic Review.Int J Mol Sci. 2022 Aug 3;23(15):8650. doi: 10.3390/ijms23158650. Int J Mol Sci. 2022. PMID: 35955775 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

