Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine, or Epstein-Barr Virus EBNA-1
- PMID: 32429322
- PMCID: PMC7281552
- DOI: 10.3390/cancers12051254
Characteristics of MGUS and Multiple Myeloma According to the Target of Monoclonal Immunoglobulins, Glucosylsphingosine, or Epstein-Barr Virus EBNA-1
Abstract
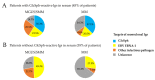
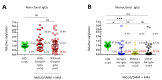
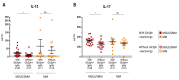
: Chronic stimulation by infectious or self-antigens initiates subsets of monoclonal gammopathies of undetermined significance (MGUS), smoldering multiple myeloma (SMM), or multiple myeloma (MM). Recently, glucosylsphingosine (GlcSph) was reported to be the target of one third of monoclonal immunoglobulins (Igs). In this study of 233 patients (137 MGUS, 6 SMM, 90 MM), we analyzed the GlcSph-reactivity of monoclonal Igs and non-clonal Igs. The presence of GlcSph-reactive Igs in serum was unexpectedly frequent, detected for 103/233 (44.2%) patients. However, GlcSph was targeted by the patient's monoclonal Ig for only 37 patients (15.9%); for other patients (44 MGUS, 22 MM), the GlcSph-reactive Igs were non-clonal. Then, the characteristics of patients were examined: compared to MM with an Epstein-Barr virus EBNA-1-reactive monoclonal Ig, MM patients with a GlcSph-reactive monoclonal Ig had a mild presentation. The inflammation profiles of patients were similar except for moderately elevated levels of 4 cytokines for patients with GlcSph-reactive Igs. In summary, our study highlights the importance of analyzing clonal Igs separately from non-clonal Igs and shows that, if autoimmune responses to GlcSph are frequent in MGUS/SMM and MM, GlcSph presumably represents the initial pathogenic event for ~16% cases. Importantly, GlcSph-initiated MM appears to be a mild form of MM disease.
Keywords: antigen specificity; auto-antigen; cytokine; glucosylsphingosine (GlcSph); inflammation; interleukin-13 (IL-13); interleukin-17 (IL-17); interleukin-26 (IL-26); lysoglucosyl-ceramide (LGL1); monoclonal gammopathy of undetermined significance (MGUS); monoclonal immunoglobulin; multiple myeloma; sialylation.
Conflict of interest statement
The authors declare that they have no conflict of interest and nothing to disclose.
Figures




References
-
- Rajkumar S.V., Dimopoulos M.A., Palumbo A., Blade J., Merlini G., Mateos M.V., Kumar S., Hillengass J., Kastritis E., Richardson P., et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

